CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis
- PMID: 16567502
- PMCID: PMC2063762
- DOI: 10.1083/jcb.200509041
CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis
Abstract
Charged MVB protein 5 (CHMP5) is a coiled coil protein homologous to the yeast Vps60/Mos10 gene and other ESCRT-III complex members, although its precise function in either yeast or mammalian cells is unknown. We deleted the CHMP5 gene in mice, resulting in a phenotype of early embryonic lethality, reflecting defective late endosome function and dysregulation of signal transduction. Chmp5-/- cells exhibit enlarged late endosomal compartments that contain abundant internal vesicles expressing proteins that are characteristic of late endosomes and lysosomes. This is in contrast to ESCRT-III mutants in yeast, which are defective in multivesicular body (MVB) formation. The degradative capacity of Chmp5-/- cells was reduced, and undigested proteins from multiple pathways accumulated in enlarged MVBs that failed to traffic their cargo to lysosomes. Therefore, CHMP5 regulates late endosome function downstream of MVB formation, and the loss of CHMP5 enhances signal transduction by inhibiting lysosomal degradation of activated receptors.
Figures
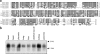
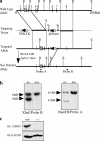
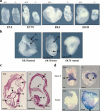
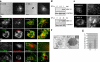


References
-
- Babst, M. 2005. A protein's final ESCRT. Traffic. 6:2–9. - PubMed
-
- Babst, M., G. Odorizzi, E.J. Estepa, and S.D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 1:248–258. - PubMed
-
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Meerloo, and S.D. Emr. 2002. a. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell. 3:271–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

