CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion
- PMID: 16567503
- PMCID: PMC2063763
- DOI: 10.1083/jcb.200508058
CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion
Abstract
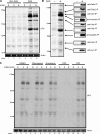
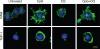
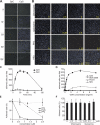
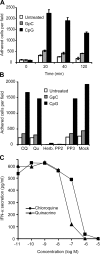
Toll-like receptors (TLRs) recognize molecular patterns preferentially expressed by pathogens. In endosomes, TLR9 is activated by unmethylated bacterial DNA, resulting in proinflammatory cytokine secretion via the adaptor protein MyD88. We demonstrate that CpG oligonucleotides activate a TLR9-independent pathway initiated by two Src family kinases, Hck and Lyn, which trigger a tyrosine phosphorylation-mediated signaling cascade. This cascade induces actin cytoskeleton reorganization, resulting in cell spreading, adhesion, and motility. CpG-induced actin polymerization originates at the plasma membrane, rather than in endosomes. Chloroquine, an inhibitor of CpG-triggered cytokine secretion, blocked TLR9/MyD88-dependent cytokine secretion as expected but failed to inhibit CpG-induced Src family kinase activation and its dependent cellular responses. Knock down of Src family kinase expression or the use of specific kinase inhibitors blocked MyD88-dependent signaling and cytokine secretion, providing evidence that tyrosine phosphorylation is both CpG induced and an upstream requirement for the engagement of TLR9. The Src family pathway intersects the TLR9-MyD88 pathway by promoting the tyrosine phosphorylation of TLR9 and the recruitment of Syk to this receptor.
Figures




References
-
- Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R.M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958–1968. - PubMed
-
- Araiza-Casillas, R., F. Cardenas, Y. Morales, and M.H. Cardiel. 2004. Factors associated with chloroquine-induced retinopathy in rheumatic diseases. Lupus. 13:119–124. - PubMed
-
- Arbibe, L., J.P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P.J. Godowski, R.J. Ulevitch, and U.G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533–540. - PubMed
-
- Baek, K.H., S.J. Ha, and Y.C. Sung. 2001. A novel function of phosphorothioate oligodeoxynucleotides as chemoattractants for primary macrophages. J. Immunol. 167:2847–2854. - PubMed
-
- Beaty, C.D., T.L. Franklin, Y. Uehara, and C.B. Wilson. 1994. Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. Eur. J. Immunol. 24:1278–1284. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

