Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells
- PMID: 16567509
- PMCID: PMC3463958
- DOI: 10.2337/diabetes.55.04.06.db05-0932
Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells
Abstract
The objective of this study was to determine whether the polyol pathway enzyme aldose reductase mediates diabetes abnormalities in vascular smooth muscle cell (SMC) growth. Aldose reductase inhibitors (tolrestat or sorbinil) or antisense aldose reductase mRNA prevented hyperproliferation of cultured rat aortic SMCs induced by high glucose. Cell cycle progression in the presence of high glucose was blocked by tolrestat, which induced a G0-G1 phase growth arrest. In situ, diabetes increased SMC growth and intimal hyperplasia in balloon-injured carotid arteries of streptozotocin-treated rats, when examined 7 or 14 days after injury. Treatment with tolrestat (15 mg x kg(-1) x day(-1)) diminished intimal hyperplasia and decreased SMC content of the lesion by 25%. Although tolrestat treatment increased immunoreactivity of the lesion with antibodies raised against protein adducts of the lipid peroxidation product 4-hydroxy trans-2-nonenal, no compensatory increase in lesion fibrosis was observed. Collectively, these results suggest that inhibition of aldose reductase prevents glucose-induced stimulation of SMC growth in culture and in situ. Even though inhibition of aldose reductase increases vascular oxidative stress, this approach may be useful in preventing abnormal SMC growth in vessels of diabetic patients.
Figures
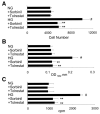
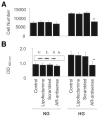





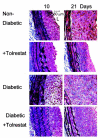

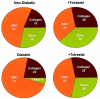
References
-
- Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. - PubMed
-
- Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. - PubMed
-
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. - PubMed
-
- Stern MP. Diabetes and cardiovascular disease: the “common soil” hypothesis. Diabetes. 1995;44:369–374. - PubMed
-
- Barsness GW, Peterson ED, Ohman EM, Nelson CL, DeLong ER, Reves JG, Smith PK, Anderson RD, Jones RH, Mark DB, Califf RM. Relationship between diabetes mellitus and long-term survival after coronary bypass and angioplasty. Circulation. 1997;96:2551–2556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

