Reelin expression and glycosylation patterns are altered in Alzheimer's disease
- PMID: 16567613
- PMCID: PMC1414634
- DOI: 10.1073/pnas.0601279103
Reelin expression and glycosylation patterns are altered in Alzheimer's disease
Abstract
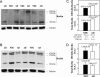
Reelin is a glycoprotein that is essential for the correct cytoarchitectonic organization of the developing CNS. Its function in the adult brain is less understood, although it has been proposed that Reelin is involved in signaling pathways linked to neurodegeneration. Here we analyzed Reelin expression in brains and cerebrospinal fluid (CSF) from Alzheimer's disease (AD) patients and nondemented controls. We found a 40% increase in the Reelin protein levels in the cortex of AD patients compared with controls. Similar increases were detected at the Reelin mRNA transcriptional level. This expression correlates with parallel increases in CSF but not in plasma samples. Next, we examined whether CSF Reelin levels were also altered in neurological diseases, including frontotemporal dementia, progressive supranuclear palsy, and Parkinson's disease. The Reelin 180-kDa band increased in all of the neurodegenerative disorders analyzed. Moreover, the 180-kDa Reelin levels correlated positively with Tau protein in CSF. Finally, we studied the pattern of Reelin glycosylation by using several lectins and the anti-HNK-1 antibody. Glycosylation differed in plasma and CSF. Furthermore, the pattern of Reelin lectin binding differed between the CSF of controls and in AD. Our results show that Reelin is up-regulated in the brain and CSF in several neurodegenerative diseases and that CSF and plasma Reelin have distinct cellular origins, thereby supporting that Reelin is involved in the pathogenesis of a number of neurodegenerative diseases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



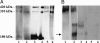
References
-
- D’Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T. Neuron. 1999;24:471–479. - PubMed
-
- Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J. Neuron. 1999;24:481–489. - PubMed
-
- Bar I., Goffinet A. M. Nature. 1999;399:645–646. - PubMed
-
- Cooper J. A., Howell B. W. Cell. 1999;97:671–674. - PubMed
-
- Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. Cell. 1999;97:689–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

