Green primaries: environmentally friendly energetic complexes
- PMID: 16567623
- PMCID: PMC1459368
- DOI: 10.1073/pnas.0600827103
Green primaries: environmentally friendly energetic complexes
Abstract
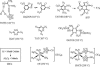
Primary explosives are used in small quantities to generate a detonation wave when subjected to a flame, heat, impact, electric spark, or friction. Detonation of the primary explosive initiates the secondary booster or main-charge explosive or propellant. Long-term use of lead azide and lead styphnate as primary explosives has resulted in lead contamination at artillery and firing ranges and become a major health hazard and environmental problem for both military and civilian personnel. Devices using lead primary explosives are manufactured by the tens of millions every year in the United States from primers for bullets to detonators for mining. Although substantial synthetic efforts have long been focused on the search for greener primary explosives, this unresolved problem has become a "holy grail" of energetic materials research. Existing candidates suffer from instability or excessive sensitivity, or they possess toxic metals or perchlorate. We report here four previously undescribed green primary explosives based on complex metal dianions and environmentally benign cations, (cat)(2)[M(II)(NT)(4)(H(2)O)(2)] (where cat is NH(4)(+) or Na(+), M is Fe(2+) or Cu(2+), and NT(-) is 5-nitrotetrazolato-N(2)). They are safer to prepare, handle, and transport than lead compounds, have comparable initiation efficiencies to lead azide, and offer rapid reliable detonation comparable with lead styphnate. Remarkably, they possess all current requirements for green primary explosives and are suitable to replace lead primary explosives in detonators. More importantly, they can be synthesized more safely, do not pose health risks to personnel, and cause much less pollution to the environment.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

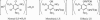
References
-
- Curtius T. Chem. Ber. 1891;24:3345–3346.
-
- Herz E. V. Beilstein. 1914;6:405.
-
- Fedoroff B. T., Sheffield O. E. Encyclopedia of Explosives and Related Items. Vol. 6. Dover, NJ: Picatinny Arsenal; 1966. pp. F217–F223.
-
- Fedoroff B. T., Sheffield O. E. Encyclopedia of Explosives and Related Items. Vol. 5. Dover, NJ: Picatinny Arsenal; 1966. p. D1588.
-
- Giles J. Nature. 2004;427:580–581. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

