Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions
- PMID: 16568366
- PMCID: PMC2504580
- DOI: 10.1007/s10162-006-0028-9
Simultaneous measurement of noise-activated middle-ear muscle reflex and stimulus frequency otoacoustic emissions
Abstract
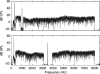
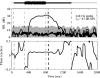
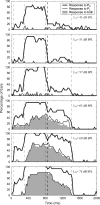
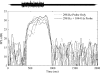
Otoacoustic emissions serve as a noninvasive probe of the medial olivocochlear (MOC) reflex. Stimulus frequency otoacoustic emissions (SFOAEs) elicited by a low-level probe tone may be the optimal type of emission for studying MOC effects because at low levels, the probe itself does not elicit the MOC reflex [Guinan et al. (2003) J. Assoc. Res. Otolaryngol. 4:521]. Based on anatomical considerations, the MOC reflex activated by ipsilateral acoustic stimulation (mediated by the crossed olivocochlear bundle) is predicted to be stronger than the reflex to contralateral stimulation. Broadband noise is an effective activator of the MOC reflex; however, it is also an effective activator of the middle-ear muscle (MEM) reflex, which can make results difficult to interpret. The MEM reflex may be activated at lower levels than measured clinically, and most previous human studies have not explicitly included measurements to rule out MEM reflex contamination. The current study addressed these issues using a higher-frequency SFOAE probe tone to test for cochlear changes mediated by the MOC reflex, while simultaneously monitoring the MEM reflex using a low-frequency probe tone. Broadband notched noise was presented ipsilaterally at various levels to elicit probe-tone shifts. Measurements are reported for 15 normal-hearing subjects. With the higher-frequency probe near 1.5 kHz, only 20% of subjects showed shifts consistent with an MOC reflex in the absence of an MEM-induced shift. With the higher-frequency probe near 3.5 kHz, up to 40% of subjects showed shifts in the absence of an MEM-induced shift. However, these responses had longer time courses than expected for MOC-induced shifts, and may have been dominated by other cochlear processes, rather than MOC reflex. These results suggest caution in the interpretation of effects observed using ipsilaterally presented acoustic activators intended to excite the MOC reflex.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

