The key regulators of adult T helper cell responses, STAT6 and T-bet, are established in early life in mice
- PMID: 16568497
- PMCID: PMC2112774
- DOI: 10.1002/eji.200535563
The key regulators of adult T helper cell responses, STAT6 and T-bet, are established in early life in mice
Abstract
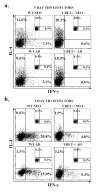
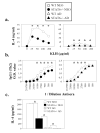
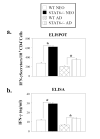
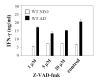
Murine neonatal immunity is typically Th2 biased. This is characterized by high-level IL-4 production at all phases of the immune response and poor IFN-gamma memory responses. The differential expression of Th1/Th2 cytokines by neonates and adults could arise if the critical regulators of Th differentiation and function, STAT6 and T-bet, operate differently during the neonatal period. To test this idea, the Th cell responses of wild-type, T-bet-deficient, or STAT6-deficient mice were compared in vitro and in vivo. The absence of these factors had similar qualitative effects on the development of effector function in neonates and adults, i.e., if a Th lineage was inhibited or enhanced in adult animals, a similar phenomenon was observed in neonates. However, there was a striking difference observed in the in vivo Th1 memory responses of STAT6-deficient mice initially immunized as neonates. Antigen-specific IFN-gamma production was increased 50-100-fold in STAT6-deficient neonates, achieving levels similar to those of STAT6-deficient adults. These findings demonstrate that STAT6 and T-bet signals are central in shaping Th responses in wild-type neonates, as in adult mice, and that the master regulators of Th cell development and function are already firmly established in early life.
Figures




Similar articles
-
Cytotoxic T-lymphocyte antigen-4 inhibits GATA-3 but not T-bet mRNA expression during T helper cell differentiation.Immunology. 2006 Mar;117(3):358-67. doi: 10.1111/j.1365-2567.2005.02309.x. Immunology. 2006. PMID: 16476055 Free PMC article.
-
Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation.J Immunol. 2003 Oct 1;171(7):3542-9. doi: 10.4049/jimmunol.171.7.3542. J Immunol. 2003. PMID: 14500650
-
Neonatal tolerance in the absence of Stat4- and Stat6- dependent Th cell differentiation.J Immunol. 2002 Oct 15;169(8):4124-8. doi: 10.4049/jimmunol.169.8.4124. J Immunol. 2002. PMID: 12370340
-
STAT4 and STAT6, their role in cellular and humoral immunity and in diverse human diseases.Int Rev Immunol. 2024;43(6):394-418. doi: 10.1080/08830185.2024.2395274. Epub 2024 Aug 26. Int Rev Immunol. 2024. PMID: 39188021 Review.
-
The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells.J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672. Epub 2014 May 13. J Immunol Res. 2014. PMID: 24901011 Free PMC article. Review.
Cited by
-
New therapeutic approaches for protecting hematopoietic stem cells in aplastic anemia.Immunol Res. 2013 Dec;57(1-3):34-43. doi: 10.1007/s12026-013-8449-0. Immunol Res. 2013. PMID: 24203441 Review.
-
Lack of Cell Cycle Inhibitor p21 and Low CD4+ T Cell Suppression in Newborns After Exposure to IFN-β.Front Immunol. 2021 Apr 12;12:652965. doi: 10.3389/fimmu.2021.652965. eCollection 2021. Front Immunol. 2021. PMID: 33912177 Free PMC article.
-
Unbalanced Neonatal CD4(+) T-Cell Immunity.Front Immunol. 2014 Aug 27;5:393. doi: 10.3389/fimmu.2014.00393. eCollection 2014. Front Immunol. 2014. PMID: 25221551 Free PMC article. Review.
-
Immune responses of female BALB/c and C57BL/6 neonatal mice to vaccination or intestinal infection are unaltered by exposure to breast milk lycopene.J Nutr. 2011 Jul;141(7):1326-30. doi: 10.3945/jn.110.136762. Epub 2011 May 18. J Nutr. 2011. PMID: 21593356 Free PMC article.
-
A novel subset of helper T cells promotes immune responses by secreting GM-CSF.Cell Death Differ. 2013 Dec;20(12):1731-41. doi: 10.1038/cdd.2013.130. Epub 2013 Sep 27. Cell Death Differ. 2013. PMID: 24076588 Free PMC article.
References
-
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. - PubMed
-
- Bot A, Antohi S, Bot S, Garcia-Sastre A, Bona C. Induction of humoral and cellular immunity against influenza virus by immunization of newborn mice with a plasmid bearing a hemagglutinin gene. Int Immunol. 1997;9:1641–1650. - PubMed
-
- Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. - PubMed
-
- Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

