IL-18 stimulates IL-13-mediated IFN-gamma-sensitive host resistance in vivo
- PMID: 16568498
- PMCID: PMC2000330
- DOI: 10.1002/eji.200535668
IL-18 stimulates IL-13-mediated IFN-gamma-sensitive host resistance in vivo
Abstract
IL-4 and IL-13 are up-regulated during in vivo responses to many nematode parasites, but increasing evidence suggests that increases in IL-13 can also occur independently of the IL-4-dominant Th2 response. Blocking B7 after Trichuris muris inoculation inhibits resistance and IL-4 elevations, instead resulting in an IFN-gamma-dominant response associated with susceptibility. However, blocking IFN-gamma under these conditions restores IL-13-dependent resistance. In this study, we examined the mechanism of IL-13 up-regulation and associated protection during this in vivo immune response. CD4+ T cells and DX5+ TCR- cells were identified as the major producers of IL-13, and the DX5+ TCR- cells were phenotyped as NK cells, since they expressed CD11b, IL-2Rbeta and Ly49C but not c-kit or Fc epsilonRI. NK cell-derived IL-13 elevations were T cell-dependent, as CD4+ T cell depletion blocked IL-13 production by mesenteric lymph node cells and induced susceptibility. IL-13 expression was increased independently of IL-12; however, blocking IL-18 function inhibited IL-13 production and increased susceptibility. These results indicate that CD4+ T cells and NK cells are the major sources of IL-13 during the in vivo Th1 response induced by B7 blockade and that under these conditions, IL-18 is specifically required for the in vivo up-regulation of IL-13 production and associated host protection.
Figures

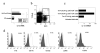

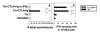


Similar articles
-
IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive.J Immunol. 2000 Apr 15;164(8):4250-6. doi: 10.4049/jimmunol.164.8.4250. J Immunol. 2000. PMID: 10754322
-
Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13.J Exp Med. 2001 Aug 6;194(3):355-64. doi: 10.1084/jem.194.3.355. J Exp Med. 2001. PMID: 11489954 Free PMC article.
-
IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells.J Immunol. 1996 Feb 15;156(4):1458-68. J Immunol. 1996. PMID: 8568248
-
B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation.J Exp Med. 1994 Jul 1;180(1):223-31. doi: 10.1084/jem.180.1.223. J Exp Med. 1994. PMID: 7516409 Free PMC article.
-
Tumor-induced suppression of interferon-gamma production and enhancement of interleukin-10 production by natural killer (NK) cells: paralleled to CD4+ T cells.Mol Immunol. 2005 May;42(9):1023-31. doi: 10.1016/j.molimm.2004.09.035. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829292
Cited by
-
Disruption of Th2 immunity results in a gender-specific expansion of IL-13 producing accessory NK cells during helminth infection.J Immunol. 2009 Sep 15;183(6):3906-14. doi: 10.4049/jimmunol.0900577. Epub 2009 Aug 19. J Immunol. 2009. PMID: 19692641 Free PMC article.
-
B cells have distinct roles in host protection against different nematode parasites.J Immunol. 2010 May 1;184(9):5213-23. doi: 10.4049/jimmunol.0902879. Epub 2010 Mar 31. J Immunol. 2010. PMID: 20357259 Free PMC article.
-
Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13.Mucosal Immunol. 2011 Jul;4(4):397-408. doi: 10.1038/mi.2010.82. Epub 2010 Dec 22. Mucosal Immunol. 2011. PMID: 21178983 Free PMC article.
-
Cathepsin L3 From Fasciola hepatica Induces NLRP3 Inflammasome Alternative Activation in Murine Dendritic Cells.Front Immunol. 2019 Mar 22;10:552. doi: 10.3389/fimmu.2019.00552. eCollection 2019. Front Immunol. 2019. PMID: 30967874 Free PMC article.
References
-
- Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. - PubMed
-
- Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, et al. Requirements for the development of IL-4-producing Tcells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57–74. - PubMed
-
- Gause WC, Urban JF, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. - PubMed
-
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. - PubMed
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

