Interferon-gamma increases neuronal death in response to amyloid-beta1-42
- PMID: 16569229
- PMCID: PMC1435873
- DOI: 10.1186/1742-2094-3-7
Interferon-gamma increases neuronal death in response to amyloid-beta1-42
Abstract
Background: Alzheimer's disease is a neurodegenerative disorder characterized by a progressive cognitive impairment, the consequence of neuronal dysfunction and ultimately the death of neurons. The amyloid hypothesis proposes that neuronal damage results from the accumulation of insoluble, hydrophobic, fibrillar peptides such as amyloid-beta1-42. These peptides activate enzymes resulting in a cascade of second messengers including prostaglandins and platelet-activating factor. Apoptosis of neurons is thought to follow as a consequence of the uncontrolled release of second messengers. Biochemical, histopathological and genetic studies suggest that pro-inflammatory cytokines play a role in neurodegeneration during Alzheimer's disease. In the current study we examined the effects of interferon (IFN)-gamma, tumour necrosis factor (TNF)alpha, interleukin (IL)-1beta and IL-6 on neurons.
Methods: Primary murine cortical or cerebellar neurons, or human SH-SY5Y neuroblastoma cells, were grown in vitro. Neurons were treated with cytokines prior to incubation with different neuronal insults. Cell survival, caspase-3 activity (a measure of apoptosis) and prostaglandin production were measured. Immunoblots were used to determine the effects of cytokines on the levels of cytoplasmic phospholipase A2 or phospholipase C gamma-1.
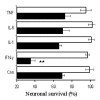
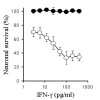
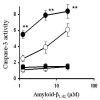
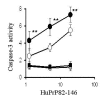
Results: While none of the cytokines tested were directly neurotoxic, pre-treatment with IFN-gamma sensitised neurons to the toxic effects of amyloid-beta1-42 or HuPrP82-146 (a neurotoxic peptide found in prion diseases). The effects of IFN-gamma were seen on cortical and cerebellar neurons, and on SH-SY5Y neuroblastoma cells. However, pre-treatment with IFN-gamma did not affect the sensitivity to neurons treated with staurosporine or hydrogen peroxide. Pre-treatment with IFN-gamma increased the levels of cytoplasmic phospholipase A2 in SH-SY5Y cells and increased prostaglandin E2 production in response to amyloid-beta1-42.
Conclusion: Treatment of neuronal cells with IFN-gamma increased neuronal death in response to amyloid-beta1-42 or HuPrP82-146. IFN-gamma increased the levels of cytoplasmic phospholipase A2 in cultured neuronal cells and increased expression of cytoplasmic phospholipase A2 was associated with increased production of prostaglandin E2 in response to amyloid-beta1-42 or HuPrP82-146. Such observations suggest that IFN-gamma produced within the brain may increase neuronal loss in Alzheimer's disease.
Figures

Similar articles
-
Ginkgolide B inhibits the neurotoxicity of prions or amyloid-beta1-42.J Neuroinflammation. 2004 May 11;1(1):4. doi: 10.1186/1742-2094-1-4. J Neuroinflammation. 2004. PMID: 15285798 Free PMC article.
-
Prostaglandin D2 mediates neuronal damage by amyloid-beta or prions which activates microglial cells.Neuropharmacology. 2006 Feb;50(2):229-37. doi: 10.1016/j.neuropharm.2005.09.008. Epub 2005 Nov 11. Neuropharmacology. 2006. PMID: 16289250
-
Docosahexaenoic and eicosapentaenoic acids increase neuronal death in response to HuPrP82-146 and Abeta 1-42.Neuropharmacology. 2008 May;54(6):934-43. doi: 10.1016/j.neuropharm.2008.02.003. Epub 2008 Feb 10. Neuropharmacology. 2008. PMID: 18355880
-
The killing of neurons by beta-amyloid peptides, prions, and pro-inflammatory cytokines.Ital J Anat Embryol. 2006 Oct-Dec;111(4):221-46. Ital J Anat Embryol. 2006. PMID: 17385278 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme.Psychopharmacology (Berl). 2008 May;198(1):1-27. doi: 10.1007/s00213-008-1092-0. Epub 2008 Feb 19. Psychopharmacology (Berl). 2008. PMID: 18392810 Review.
-
Neuregulin-1 elicits a regulatory immune response following traumatic spinal cord injury.J Neuroinflammation. 2018 Feb 21;15(1):53. doi: 10.1186/s12974-018-1093-9. J Neuroinflammation. 2018. PMID: 29467001 Free PMC article.
-
Identification of neurotoxic cytokines by profiling Alzheimer's disease tissues and neuron culture viability screening.Sci Rep. 2015 Nov 13;5:16622. doi: 10.1038/srep16622. Sci Rep. 2015. PMID: 26564777 Free PMC article.
-
Amyloid beta and diabetic pathology cooperatively stimulate cytokine expression in an Alzheimer's mouse model.J Neuroinflammation. 2020 Jan 28;17(1):38. doi: 10.1186/s12974-020-1707-x. J Neuroinflammation. 2020. PMID: 31992349 Free PMC article.
-
Metabolic Enhancer Piracetam Attenuates the Translocation of Mitochondrion-Specific Proteins of Caspase-Independent Pathway, Poly [ADP-Ribose] Polymerase 1 Up-regulation and Oxidative DNA Fragmentation.Neurotox Res. 2018 Aug;34(2):198-219. doi: 10.1007/s12640-018-9878-2. Epub 2018 Mar 12. Neurotox Res. 2018. PMID: 29532444
References
-
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989;245:417–420. - PubMed
-
- Forloni G, Chiesa R, Smiroldo S, Verga L, Salmona M, Tagliavini F, Angeretti N. Apoptosis mediated neurotoxicity induced by chronic application of beta amyloid fragment 25–35. Neuroreport. 1993;4:523–526. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

