Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma
- PMID: 16569595
- PMCID: PMC1913933
- DOI: 10.1016/j.exphem.2006.01.008
Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma
Abstract
Objective: The aim of this study was identification of human leukocyte antigen (HLA)-A2-restricted T-cell epitopes within the HM1.24 antigen as target for multiple myeloma (MM)-directed specific peptide-based immunotherapy.
Methods: The HM1.24 sequence was scanned for immunogenic peptides using the HLA-binding prediction software SYFPEITHI and BIMAS. Peripheral blood mononuclear cells from HLA-A2(+) healthy volunteers/blood donors (ND) were stimulated with autologous HM1.24-peptide-loaded dendritic cells, and expanded in vitro. Activation of T cells was assessed by ELISpot and cytotoxicity by (51)Chromium ((51)Cr)-release assays. T2-cells pulsed with irrelevant peptide, the HM1.24(-)/HLA-A2(+) breast carcinoma cell line MCF-7 and the HM1.24(+)/HLA-A2(-) myeloma cell line RPMI-8226 were used as controls. Expression of the HM1.24 gene (BST2) was assessed using purified plasma cells and Affymetrix-U133A+B microarrays. Frequency of peptide-specific CD8(+) T cells was detected using the flow-cytometric tetramer technique.
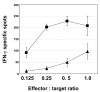
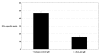
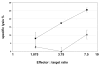
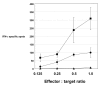
Results: Of eight nona-peptides with the highest probability of binding to HLA-A2, the HM1.24 aa22-30 peptide (LLLGIGILV) showed the most frequent activation of CD8(+) T cells in healthy volunteers (specific activation in 8 of 11 [73%] ND; compared with 5-19% for the 7 other HM1.24 peptides). Antigen recognition by the HM1.24 aa22-30-specific CD8(+) T cells was HLA-A2-restricted (ELISpot with HLA-A2-blocking antibodies: median, 15; range, 14-18 spots/well; isotype-control antibodies: median, 47; range, 44-48). HM1.24-aa22-30-specific CD8(+) T cells lysed HLA-A2(+) myeloma-derived cell lines ((51)Cr-release assay: XG-1 vs MCF-7, 91% vs 0%; U266 vs MCF-7, 38% vs 4.2%; IM-9 vs RPMI-8226, 22% vs 0%). Using the cross-reactive Neisseria meningitidis peptide LLSLGIGILV-specific CD8(+) T cells recognizing target cells loaded with the HM1.24 aa22-30 peptide (LLLGIGILV) as well as the myeloma-derived cell line U266 could be expanded from MM patients. The HM1.24 gene was expressed at comparable levels by plasma cells from 65 MM patients, 7 patients with monoclonal gammopathy of undetermined significance, and 7 ND.
Conclusions: HM1.24 aa22-30 is a newly identified HLA-A2-restricted T-cell epitope that is processed and presented by major histocompatibility complex class I. Specifically activated CD8(+) T cells are able to lyse MM cell lines. We conclude that HM1.24 aa22-30 represents a suitable candidate target for a specific peptide-based immunotherapy of MM.
Figures



Similar articles
-
Identification of HLA-A2 restricted T-cell epitopes within the conserved region of the immunoglobulin G heavy-chain in patients with multiple myeloma.Eur J Haematol. 2008 Jul;81(1):26-35. doi: 10.1111/j.1600-0609.2008.01076.x. Epub 2008 Mar 19. Eur J Haematol. 2008. PMID: 18363871 Free PMC article.
-
Melan-A/MART1 analog peptide triggers anti-myeloma T-cells through crossreactivity with HM1.24.J Immunother. 2009 Jul-Aug;32(6):613-21. doi: 10.1097/CJI.0b013e3181a95198. J Immunother. 2009. PMID: 19483648
-
Induction of HM1.24 peptide-specific cytotoxic T lymphocytes by using peripheral-blood stem-cell harvests in patients with multiple myeloma.Blood. 2005 Nov 15;106(10):3538-45. doi: 10.1182/blood-2005-04-1438. Epub 2005 Jul 21. Blood. 2005. PMID: 16037388
-
Immunotherapy in multiple myeloma: Id-specific strategies suggested by studies in animal models.Cancer Immunol Immunother. 2004 Sep;53(9):759-69. doi: 10.1007/s00262-004-0504-1. Epub 2004 Apr 15. Cancer Immunol Immunother. 2004. PMID: 15088126 Free PMC article. Review.
-
Unraveling CD8+ T cell alloimmunity: Insights into the direct pathway of antigen recognition from modern experimental tools.Am J Transplant. 2025 May 13:S1600-6135(25)00263-1. doi: 10.1016/j.ajt.2025.05.009. Online ahead of print. Am J Transplant. 2025. PMID: 40373878 Review.
Cited by
-
Immunotherapy using dendritic cells against multiple myeloma: how to improve?Clin Dev Immunol. 2012;2012:397648. doi: 10.1155/2012/397648. Epub 2012 Mar 15. Clin Dev Immunol. 2012. PMID: 22481968 Free PMC article. Review.
-
Bone marrow stromal cell antigen 2: Tumor biology, signaling pathway and therapeutic targeting (Review).Oncol Rep. 2024 Mar;51(3):45. doi: 10.3892/or.2024.8704. Epub 2024 Jan 19. Oncol Rep. 2024. PMID: 38240088 Free PMC article. Review.
-
Identification of HLA-A2 restricted T-cell epitopes within the conserved region of the immunoglobulin G heavy-chain in patients with multiple myeloma.Eur J Haematol. 2008 Jul;81(1):26-35. doi: 10.1111/j.1600-0609.2008.01076.x. Epub 2008 Mar 19. Eur J Haematol. 2008. PMID: 18363871 Free PMC article.
-
Umbilical cord blood-derived CD11c(+) dendritic cells could serve as an alternative allogeneic source of dendritic cells for cancer immunotherapy.Stem Cell Res Ther. 2015 Sep 25;6:184. doi: 10.1186/s13287-015-0160-8. Stem Cell Res Ther. 2015. PMID: 26407613 Free PMC article.
-
Immunogenic targets for specific immunotherapy in multiple myeloma.Clin Dev Immunol. 2012;2012:820394. doi: 10.1155/2012/820394. Epub 2012 May 7. Clin Dev Immunol. 2012. PMID: 22611422 Free PMC article. Review.
References
-
- Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. - PubMed
-
- Bakkus MH, Bouko Y, Samson D, et al. Post-transplantation tumour load in bone marrow, as assessed by quantitative ASO-PCR, is a prognostic parameter in multiple myeloma. Br J Haematol. 2004;126:665–74. - PubMed
-
- Bergenbrant S, Yi Q, Osterborg A, Bjorkholm M, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92:840–846. - PubMed
-
- Bjorkstrand B, Ljungman P, Bird JM, et al. Autologous stem cell transplantation in multiple myeloma: results of the European Group for Bone Marrow Transplantation. Stem Cells. 1995;13(Suppl 2):140–146. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

