A simple artificial light-harvesting dyad as a model for excess energy dissipation in oxygenic photosynthesis
- PMID: 16569703
- PMCID: PMC1414798
- DOI: 10.1073/pnas.0508530103
A simple artificial light-harvesting dyad as a model for excess energy dissipation in oxygenic photosynthesis
Abstract
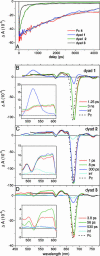
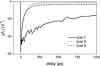
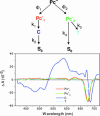
Under excess illumination, plant photosystem II dissipates excess energy through the quenching of chlorophyll fluorescence, a process known as nonphotochemical quenching. Activation of nonphotochemical quenching has been linked to the conversion of a carotenoid with a conjugation length of nine double bonds (violaxanthin) into an 11-double-bond carotenoid (zeaxanthin). It has been suggested that the increase in the conjugation length turns the carotenoid from a nonquencher into a quencher of chlorophyll singlet excited states, but unequivocal evidence is lacking. Here, we present a transient absorption spectroscopic study on a model system made up of a zinc phthalocyanine (Pc) molecule covalently linked to carotenoids with 9, 10, or 11 conjugated carbon-carbon double bonds. We show that a carotenoid can act as an acceptor of Pc excitation energy, thereby shortening its singlet excited-state lifetime. The conjugation length of the carotenoid is critical to the quenching process. Remarkably, the addition of only one double bond can turn the carotenoid from a nonquencher into a very strong quencher. By studying the solvent polarity dependence of the quenching using target analysis of the time-resolved data, we show that the quenching proceeds through energy transfer from the excited Pc to the optically forbidden S(1) state of the carotenoid, coupled to an intramolecular charge-transfer state. The mechanism for excess energy dissipation in photosystem II is discussed in view of the insights obtained on this simple model system.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Energy transfer, excited-state deactivation, and exciplex formation in artificial caroteno-phthalocyanine light-harvesting antennas.J Phys Chem B. 2007 Jun 21;111(24):6868-77. doi: 10.1021/jp071010q. Epub 2007 May 16. J Phys Chem B. 2007. PMID: 17503804
-
Dual Singlet Excited-State Quenching Mechanisms in an Artificial Caroteno-Phthalocyanine Light Harvesting Antenna.ACS Phys Chem Au. 2022 Jan 26;2(1):59-67. doi: 10.1021/acsphyschemau.1c00008. Epub 2021 Oct 14. ACS Phys Chem Au. 2022. PMID: 35098245 Free PMC article.
-
Quenching of chlorophyll fluorescence in the major light-harvesting complex of photosystem II: a systematic study of the effect of carotenoid structure.Proc Natl Acad Sci U S A. 1996 Feb 20;93(4):1492-7. doi: 10.1073/pnas.93.4.1492. Proc Natl Acad Sci U S A. 1996. PMID: 11607629 Free PMC article.
-
Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence.J Photochem Photobiol B. 1996 Oct;36(1):3-15. doi: 10.1016/S1011-1344(96)07397-6. J Photochem Photobiol B. 1996. PMID: 8988608 Review.
-
Quantum chemical insights in energy dissipation and carotenoid radical cation formation in light harvesting complexes.Phys Chem Chem Phys. 2007 Jun 21;9(23):2917-31. doi: 10.1039/b703028b. Epub 2007 Apr 25. Phys Chem Chem Phys. 2007. PMID: 17551615 Review.
Cited by
-
Engineering pH-responsive switching of donor-π-acceptor chromophore alignments along a peptide nanotube scaffold.RSC Adv. 2020 Jan 22;10(6):3588-3592. doi: 10.1039/d0ra00231c. eCollection 2020 Jan 16. RSC Adv. 2020. PMID: 35497746 Free PMC article.
-
Molecular Origin of Photoprotection in Cyanobacteria Probed by Watermarked Femtosecond Stimulated Raman Spectroscopy.J Phys Chem Lett. 2018 Apr 5;9(7):1788-1792. doi: 10.1021/acs.jpclett.8b00663. Epub 2018 Mar 26. J Phys Chem Lett. 2018. PMID: 29569927 Free PMC article.
-
Energy conversion in natural and artificial photosynthesis.Chem Biol. 2010 May 28;17(5):434-47. doi: 10.1016/j.chembiol.2010.05.005. Chem Biol. 2010. PMID: 20534342 Free PMC article. Review.
-
A two-photon excitation study on the role of carotenoid dark states in the regulation of plant photosynthesis.Photosynth Res. 2006 Nov;90(2):101-10. doi: 10.1007/s11120-006-9088-2. Epub 2007 Jan 9. Photosynth Res. 2006. PMID: 17211584
-
Triplet state dynamics in peridinin-chlorophyll-a-protein: a new pathway of photoprotection in LHCs?Biophys J. 2007 Sep 15;93(6):2118-28. doi: 10.1529/biophysj.107.106674. Epub 2007 May 4. Biophys J. 2007. PMID: 17483182 Free PMC article.
References
-
- Polivka T., Sundström V. Chem. Rev. 2004;104:2021–2071. - PubMed
-
- Frank H. A., Cogdell R. J. Photochem. Photobiol. 1996;63:257–264. - PubMed
-
- Griffiths M., Sistrom W. R., Cohenbazire G., Stanier R. Y. Nature. 1955;176:1211–1214. - PubMed
-
- Robert B., Horton P., Pascal A. A., Ruban A. V. Trends Plant Sci. 2004;9:385–390. - PubMed
-
- Holt N. E., Fleming G. R., Niyogi K. K. Biochemistry. 2004;43:8281–8289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

