Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence
- PMID: 16569765
- PMCID: PMC1895862
- DOI: 10.1182/blood-2006-01-0067
Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence
Abstract
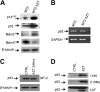
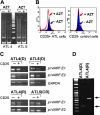
The antiviral thymidine analog azidothymidine (AZT) is used to treat several virus-associated human cancers. However, to date the mechanism of AZT action remains unclear and thus, reasons for treatment failure are unknown. Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy of poor prognosis. Here, we report that enduring AZT treatment of T-cell leukemia virus I-infected cells, in vitro and in vivo in ATL patients, results in inhibition of telomerase activity, progressive telomere shortening, and increased p14(ARF) expression. In turn, this elicits stabilization and reactivation of the tumor suppressor p53-dependent transcription, increased expression of the cyclin-dependent kinase inhibitor p21(Waf1), and accumulation of p27(kip1), thereby inducing cellular senescence and tumor cell death. While ATL patients carrying a wild-type p53 enter remission following treatment with AZT, those with a mutated p53 did not respond, and patients' disease relapse was associated with the selection of a tumor clone carrying mutated inactive p53.
Figures




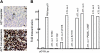
References
-
- Yoshida M. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu Rev Immunol. 2001;19: 475-496. - PubMed
-
- Bazarbachi A, Ghez D, Lepelletier Y, et al. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 2004;5: 664-672. - PubMed
-
- Hermine O, Bouscary D, Gessain A, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332: 1749-1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

