Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation
- PMID: 16569857
- PMCID: PMC1426958
- DOI: 10.1128/AAC.50.4.1393-1401.2006
Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation
Abstract
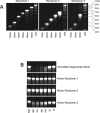
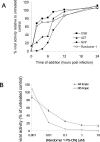
Several studies have shown that phosphorothioate oligodeoxynucleotides (PS-ONs) have a sequence-independent antiviral activity against human immunodeficiency virus type 1 (HIV-1). It has also been suggested that PS-ONs inhibit HIV-1 by acting as attachment inhibitors that bind to the V3 loop of gp120 and prevent the gp120-CD4 interaction. Here we show that PS-ONs (and their fully 2'-O-methylated derivatives) are potent inhibitors of HIV-1-mediated membrane fusion and HIV-1 replication in a size-dependent, phosphorothioation-dependent manner. PS-ONs interact with a peptide derived from the N-terminal heptad repeat region of gp41, and the HIV-1 fusion-inhibitory activity of PS-ONs is closely correlated with their ability to block gp41 six-helix bundle formation, a critical step during the process of HIV-1 fusion with the target cell. These results suggest that the increased hydrophobicity of PS-ONs may contribute to their inhibitory activity against HIV-1 fusion and entry, because longer PS-ONs (>or=30 bases) which have a greater hydrophobicity are more potent in blocking the hydrophobic interactions involved in the gp41 six-helix bundle formation and inhibiting the HIV-1-mediated cell-cell fusion than shorter PS-ONs (<30 bases). This novel antiviral mechanism of action of long PS-ONs has implications for therapy against infection by HIV-1 and other enveloped viruses with type I fusion proteins.
Figures



Similar articles
-
The stability of the intact envelope glycoproteins is a major determinant of sensitivity of HIV/SIV to peptidic fusion inhibitors.J Mol Biol. 2004 Jun 25;340(1):9-14. doi: 10.1016/j.jmb.2004.04.027. J Mol Biol. 2004. PMID: 15184018
-
HIV-1 gp41: mediator of fusion and target for inhibition.AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review.
-
N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion.Antimicrob Agents Chemother. 2004 Nov;48(11):4349-59. doi: 10.1128/AAC.48.11.4349-4359.2004. Antimicrob Agents Chemother. 2004. PMID: 15504864 Free PMC article.
-
Covalent fusion inhibitors targeting HIV-1 gp41 deep pocket.Amino Acids. 2013 Feb;44(2):701-13. doi: 10.1007/s00726-012-1394-8. Epub 2012 Sep 9. Amino Acids. 2013. PMID: 22961335
-
Small-molecule HIV-1 entry inhibitors targeting gp120 and gp41: a patent review (2010-2015).Expert Opin Ther Pat. 2017 Jun;27(6):707-719. doi: 10.1080/13543776.2017.1281249. Epub 2017 Jan 19. Expert Opin Ther Pat. 2017. PMID: 28076686 Review.
Cited by
-
Inhibition of hepatitis B viral entry by nucleic acid polymers in HepaRG cells and primary human hepatocytes.PLoS One. 2017 Jun 21;12(6):e0179697. doi: 10.1371/journal.pone.0179697. eCollection 2017. PLoS One. 2017. PMID: 28636622 Free PMC article.
-
Is There a Role for Immunoregulatory and Antiviral Oligonucleotides Acting in the Extracellular Space? A Review and Hypothesis.Int J Mol Sci. 2022 Nov 23;23(23):14593. doi: 10.3390/ijms232314593. Int J Mol Sci. 2022. PMID: 36498932 Free PMC article. Review.
-
Natural Products Mediated Targeting of Virally Infected Cancer.Dose Response. 2019 Jan 8;17(1):1559325818813227. doi: 10.1177/1559325818813227. eCollection 2019 Jan-Mar. Dose Response. 2019. PMID: 30670935 Free PMC article. Review.
-
Single-Stranded Oligonucleotide-Mediated Inhibition of Respiratory Syncytial Virus Infection.Front Immunol. 2020 Dec 8;11:580547. doi: 10.3389/fimmu.2020.580547. eCollection 2020. Front Immunol. 2020. PMID: 33363532 Free PMC article.
-
Multiple Regions Drive Hepatitis Delta Virus Proliferation and Are Therapeutic Targets.Front Microbiol. 2022 Apr 6;13:838382. doi: 10.3389/fmicb.2022.838382. eCollection 2022. Front Microbiol. 2022. PMID: 35464929 Free PMC article. Review.
References
-
- Agrawal, S., J. Y. Tang, and D. M. Brown. 1990. Analytical study of phosphorothioate analogues of oligodeoxynucleotides using high performance liquid chromatography. J. Chromatogr. 509:396-399. - PubMed
-
- Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. - PubMed
-
- Bullough, P. A., F. M. Hughson, A. C. Treharne, R. W. Ruigrok, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. - PubMed
-
- Catasti, P., E. M. Bradbury, and G. Gupta. 1996. Structure and polymorphisms of HIV third variable loops. J. Biol. Chem. 271:8236-8242. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

