Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies
- PMID: 16569864
- PMCID: PMC1426925
- DOI: 10.1128/AAC.50.4.1449-1457.2006
Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies
Abstract
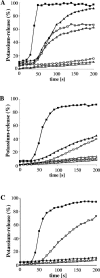
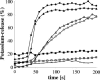
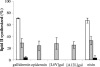
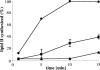
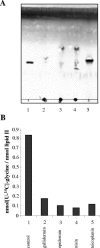
The activity of lanthionine-containing peptide antibiotics (lantibiotics) is based on different killing mechanisms which may be combined in one molecule. The prototype lantibiotic nisin inhibits peptidoglycan synthesis and forms pores through specific interaction with the cell wall precursor lipid II. Gallidermin and epidermin possess the same putative lipid II binding motif as nisin; however, both peptides are considerably shorter (22 amino acids, compared to 34 in nisin). We demonstrate that in model membranes, lipid II-mediated pore formation by gallidermin depends on membrane thickness. With intact cells, pore formation was less pronounced than for nisin and occurred only in some strains. In Lactococcus lactis subsp. cremoris HP, gallidermin was not able to release K+, and a mutant peptide, [A12L]gallidermin, in which the ability to form pores was disrupted, was as potent as wild-type gallidermin, indicating that pore formation does not contribute to killing. In contrast, nisin rapidly formed pores in the L. lactis strain; however, it was approximately 10-fold less effective in killing. The superior activity of gallidermin in a cell wall biosynthesis assay may help to explain this high potency. Generally, it appears that the multiple activities of lantibiotics combine differently for individual target strains.
Figures
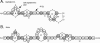

Similar articles
-
Membrane lipids determine the antibiotic activity of the lantibiotic gallidermin.J Membr Biol. 2008 Nov-Dec;226(1-3):9-16. doi: 10.1007/s00232-008-9134-4. Epub 2008 Nov 14. J Membr Biol. 2008. PMID: 19009315
-
Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors.Microb Drug Resist. 2012 Jun;18(3):261-70. doi: 10.1089/mdr.2011.0242. Epub 2012 Mar 20. Microb Drug Resist. 2012. PMID: 22432708
-
The mode of action of the lantibiotic lacticin 3147--a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II.Mol Microbiol. 2006 Jul;61(2):285-96. doi: 10.1111/j.1365-2958.2006.05223.x. Epub 2006 Jun 12. Mol Microbiol. 2006. PMID: 16771847
-
Antimicrobial mechanism of lantibiotics.Biochem Soc Trans. 2012 Dec 1;40(6):1528-33. doi: 10.1042/BST20120190. Biochem Soc Trans. 2012. PMID: 23176511 Review.
-
A lesson in efficient killing from two-component lantibiotics.Mol Microbiol. 2006 Jul;61(2):271-3. doi: 10.1111/j.1365-2958.2006.05239.x. Epub 2006 Jun 12. Mol Microbiol. 2006. PMID: 16771845 Review.
Cited by
-
Bacteriocin diversity in Streptococcus and Enterococcus.J Bacteriol. 2007 Feb;189(4):1189-98. doi: 10.1128/JB.01254-06. Epub 2006 Nov 10. J Bacteriol. 2007. PMID: 17098898 Free PMC article. Review. No abstract available.
-
Immunomodulatory and Allergenic Properties of Antimicrobial Peptides.Int J Mol Sci. 2022 Feb 24;23(5):2499. doi: 10.3390/ijms23052499. Int J Mol Sci. 2022. PMID: 35269641 Free PMC article. Review.
-
Systematic characterization of position one variants within the lantibiotic nisin.Sci Rep. 2019 Jan 30;9(1):935. doi: 10.1038/s41598-018-37532-4. Sci Rep. 2019. PMID: 30700815 Free PMC article.
-
Insights into the mode of action of chitosan as an antibacterial compound.Appl Environ Microbiol. 2008 Jun;74(12):3764-73. doi: 10.1128/AEM.00453-08. Epub 2008 May 2. Appl Environ Microbiol. 2008. PMID: 18456858 Free PMC article.
-
The lantibiotic gallidermin acts bactericidal against Staphylococcus epidermidis and Staphylococcus aureus and antagonizes the bacteria-induced proinflammatory responses in dermal fibroblasts.Microbiologyopen. 2018 Dec;7(6):e00606. doi: 10.1002/mbo3.606. Epub 2018 Mar 13. Microbiologyopen. 2018. PMID: 29536668 Free PMC article.
References
-
- Allgaier, H., G. Jung, R. G. Werner, U. Schneider, and H. Zahner. 1986. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur. J. Biochem. 160:9-22. - PubMed
-
- Benz, R., G. Jung, and H. G. Sahl. 1991. Mechanism of channel-formation by lantibiotics in black lipid membranes, p. 359-372. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
-
- Bhattacharya, S., and S. Haldar. 2000. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochim. Biophys. Acta 1467:39-53. - PubMed
-
- Bierbaum, G., and H. G. Sahl. 1985. Induction of autolysis of staphylococci by the basic peptide antibiotic Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch. Microbiol. 141:249-254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

