Preclinical evaluation of synthetic -2 RANTES as a candidate vaginal microbicide to target CCR5
- PMID: 16569870
- PMCID: PMC1426989
- DOI: 10.1128/AAC.50.4.1497-1509.2006
Preclinical evaluation of synthetic -2 RANTES as a candidate vaginal microbicide to target CCR5
Abstract
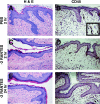
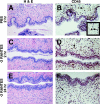
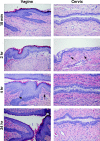
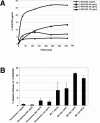
A potential strategy that can be used to combat the worldwide AIDS epidemic is the development of a vaginal microbicide that prevents the sexual transmission of human immunodeficiency virus type 1 (HIV-1). Certain CC chemokines, including RANTES, MIP-1alpha, and MIP-1beta, might facilitate the development of such microbicides since they potently suppress HIV-1 infection by binding to CCR5, the viral coreceptor used by most sexually transmitted strains of HIV-1 to enter host cells. In this study, we evaluated whether a CCR5-specific fragment of RANTES that lacks two N-terminal residues (-2 RANTES) and possesses especially potent HIV-1 suppressive activity has toxicity profiles conducive to the advancement of testing in candidate microbicide formulations. Analyses were carried out with a synthetic version of the chemokine, which was formulated with either Novasomes 7474, a nonphospholipid liposome, or methylcellulose gel. Dialysis studies demonstrated that the formulated -2 RANTES was released from both vehicles and retained anti-HIV-1 activity. Preclinical toxicity studies carried out with Swiss Webster mouse and New Zealand White rabbit vaginal irritation models demonstrated minimal inflammation and minimal adverse changes in cervicovaginal tissue integrity after short-term (10 min) and long-term (24 h) exposure to formulations containing up to 1 mg/ml of -2 RANTES. Similarly, no toxicity was observed with formulations of bioactive murine RANTES in the Swiss Webster mouse vaginal irritation model. Overall, these preclinical studies suggest that -2 RANTES is suitable for further testing as a candidate anti-HIV-1 microbicide.
Figures


Similar articles
-
Evaluation of -2 RANTES vaginal microbicide formulations in a nonhuman primate simian/human immunodeficiency virus (SHIV) challenge model.AIDS Res Hum Retroviruses. 2007 Jan;23(1):33-42. doi: 10.1089/aid.2006.0076. AIDS Res Hum Retroviruses. 2007. PMID: 17263630
-
Pharmacokinetics of the Protein Microbicide 5P12-RANTES in Sheep following Single-Dose Vaginal Gel Administration.Antimicrob Agents Chemother. 2017 Sep 22;61(10):e00965-17. doi: 10.1128/AAC.00965-17. Print 2017 Oct. Antimicrob Agents Chemother. 2017. PMID: 28784672 Free PMC article.
-
PSC-RANTES blocks R5 human immunodeficiency virus infection of Langerhans cells isolated from individuals with a variety of CCR5 diplotypes.J Virol. 2004 Jul;78(14):7602-9. doi: 10.1128/JVI.78.14.7602-7609.2004. J Virol. 2004. PMID: 15220435 Free PMC article.
-
Rational design of novel HIV-1 entry inhibitors by RANTES engineering.Vaccine. 2008 Jun 6;26(24):3008-15. doi: 10.1016/j.vaccine.2007.12.023. Epub 2008 Jan 10. Vaccine. 2008. PMID: 18243436 Free PMC article. Review.
-
Microbicides: a new hope for HIV prevention.Indian J Med Res. 2011 Dec;134(6):939-49. doi: 10.4103/0971-5916.92639. Indian J Med Res. 2011. PMID: 22310826 Free PMC article. Review.
Cited by
-
Pre-clinical development as microbicide of zinc tetra-ascorbo-camphorate, a novel terpenoid derivative: potent in vitro inhibitory activity against both R5- and X4-tropic HIV-1 strains without significant in vivo mucosal toxicity.AIDS Res Ther. 2008 Jun 3;5:10. doi: 10.1186/1742-6405-5-10. AIDS Res Ther. 2008. PMID: 18522743 Free PMC article.
-
Accurate structure prediction of peptide-MHC complexes for identifying highly immunogenic antigens.Mol Immunol. 2013 Nov;56(1-2):81-90. doi: 10.1016/j.molimm.2013.04.011. Epub 2013 May 18. Mol Immunol. 2013. PMID: 23688437 Free PMC article.
-
Microbicide safety/efficacy studies in animals: macaques and small animal models.Curr Opin HIV AIDS. 2008 Sep;3(5):567-73. doi: 10.1097/COH.0b013e32830891bb. Curr Opin HIV AIDS. 2008. PMID: 19373023 Free PMC article.
-
Griffithsin Retains Anti-HIV-1 Potency with Changes in gp120 Glycosylation and Complements Broadly Neutralizing Antibodies PGT121 and PGT126.Antimicrob Agents Chemother. 2019 Dec 20;64(1):e01084-19. doi: 10.1128/AAC.01084-19. Print 2019 Dec 20. Antimicrob Agents Chemother. 2019. PMID: 31611356 Free PMC article.
-
Distinct efficacy of HIV-1 entry inhibitors to prevent cell-to-cell transfer of R5 and X4 viruses across a human placental trophoblast barrier in a reconstitution model in vitro.Retrovirology. 2008 Mar 31;5:31. doi: 10.1186/1742-4690-5-31. Retrovirology. 2008. PMID: 18377645 Free PMC article.
References
-
- Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. - PubMed
-
- Reference deleted.
-
- Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. - PMC - PubMed
-
- American Physiological Society. 1996. Guiding principles in the care and use of animals. National Academy Press, Washington, D.C.
-
- AVMA Panel on Euthanasia. 2001. 2000 report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 218:669-696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

