Phase I/II study of S-1 combined with irinotecan for metastatic advanced gastric cancer
- PMID: 16570038
- PMCID: PMC2361252
- DOI: 10.1038/sj.bjc.6603072
Phase I/II study of S-1 combined with irinotecan for metastatic advanced gastric cancer
Abstract
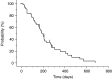
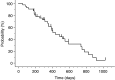
A dose-escalation study of irinotecan (CPT-11) combined with S-1, an oral dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, was performed to determine the maximum-tolerated dose (MTD), recommended dose (RD), dose-limiting toxicities (DLTs), and objective response rate (RR) in advanced gastric cancer (AGC). S-1 was administered orally at 80 mg m-2 day-1 from day 1 to 14 of a 28-day cycle and CPT-11 was given intravenously on day 1 and 8 at an initial dose of 70 mg m-2 day-1, stepping up to 100 mg m-2. The treatment was repeated every 4 weeks, unless disease progression was observed. In the phase I portion, the MTD of CPT-11 was presumed to be 100 mg m-2, because 66.6% of patients (two of three) developed DLTs. All three patients at the initial RD of CPT-11 (90 mg m-2) experienced grade 4 haematological or grade 3 nonhaematological toxicities at second course, followed by the dose reduction of CPT-11 from the third course. Considering safety and the ability to continue treatment, the final RD was determined to be 80 mg m-2. In the phase II portion, 42 patients including seven patients in the final RD phase I portion were evaluated. The median treatment course was five (range: 1-13). The incidences of severe (grade 3-4) haematological and nonhaematological toxicities were 19 and 10%, respectively, but all were manageable. The RR was 62% (26 of 42, 95% confidence interval: 47.2-76.6%), and the median survival time was 444 days. Our phase I/II trial showed S-1 combined with CPT-11 is effective for AGC and is well tolerated, with acceptable toxicity.
Figures
Similar articles
-
Phase I study of S-1 combined with irinotecan (CPT-11) in patients with advanced gastric cancer (OGSG 0002).Jpn J Clin Oncol. 2005 Sep;35(9):520-5. doi: 10.1093/jjco/hyi148. Epub 2005 Sep 1. Jpn J Clin Oncol. 2005. PMID: 16141295 Clinical Trial.
-
Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer.Br J Cancer. 2003 Dec 15;89(12):2207-12. doi: 10.1038/sj.bjc.6601413. Br J Cancer. 2003. PMID: 14676796 Free PMC article. Clinical Trial.
-
[Combination chemotherapy of TS-1 +cisplatin for inoperable gastric cancer].Gan To Kagaku Ryoho. 2004 Nov;31(12):1957-61. Gan To Kagaku Ryoho. 2004. PMID: 15570920 Review. Japanese.
-
Dosage escalation study of S-1 and irinotecan in metronomic chemotherapy against advanced colorectal cancer.Kurume Med J. 2009;56(1-2):1-7. doi: 10.2739/kurumemedj.56.1. Kurume Med J. 2009. PMID: 20103995 Clinical Trial.
-
[The current evidence of S-1 and irinotecan hydrochloride combination chemotherapy with tri-weekly schedule].Gan To Kagaku Ryoho. 2006 Jun;33 Suppl 1:125-30. doi: 10.2217/14750708.3.1.125. Gan To Kagaku Ryoho. 2006. PMID: 16897987 Review. Japanese.
Cited by
-
Gastrojejunostomy as induction treatment for S-1-based chemotherapy in patients with incurable gastric cancer.Surg Today. 2008;38(12):1102-7. doi: 10.1007/s00595-007-3749-4. Epub 2008 Nov 28. Surg Today. 2008. PMID: 19039635
-
Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer.World J Gastrointest Oncol. 2010 Feb 15;2(2):85-97. doi: 10.4251/wjgo.v2.i2.85. World J Gastrointest Oncol. 2010. PMID: 21160926 Free PMC article.
-
A hierarchical Bayesian design for phase I trials of novel combinations of cancer therapeutic agents.Biometrics. 2010 Sep;66(3):805-12. doi: 10.1111/j.1541-0420.2009.01363.x. Biometrics. 2010. PMID: 19995354 Free PMC article.
-
REDOMA: Bayesian random-effects dose-optimization meta-analysis using spike-and-slab priors.Stat Med. 2024 Aug 15;43(18):3484-3502. doi: 10.1002/sim.10107. Epub 2024 Jun 10. Stat Med. 2024. PMID: 38857904 Free PMC article.
-
Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002).Gastric Cancer. 2011 Mar;14(1):72-80. doi: 10.1007/s10120-011-0009-5. Epub 2011 Feb 23. Gastric Cancer. 2011. PMID: 21340666 Free PMC article. Clinical Trial.
References
-
- Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF (1998) Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am 4: 269–274 - PubMed
-
- Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, Hill ME, Norman AR (2004) Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 15: 64–69 - PubMed
-
- Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, Sakata Y, Hyodo I (1999) Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 17: 319–323 - PubMed
-
- Bouche O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G, Arsene D, Paitel JF, Guerin-Meyer V, Mitry E, Buecher B, Kaminsky MC, Seitz JF, Rougier P, Bedenne L, Milan C (2004) Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study – FFCD 9803. J Clin Oncol 22: 4319–4328 - PubMed
-
- Cao S, Rustum YM (2000) Synergistic antitumour activity of irinotecan in combination with 5-fluorouracil in rats bearing advanced colorectal cancer: role of drug sequence and dose. Cancer Res 60: 3717–3721 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous