COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer
- PMID: 16570043
- PMCID: PMC2361247
- DOI: 10.1038/sj.bjc.6603067
COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer
Abstract
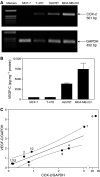
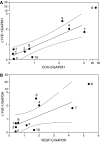
Increased expression of COX-2 or VEGF-C has been correlated with progressive disease in certain cancers. Present study utilized several human breast cancer cell lines (MCF-7, T-47D, Hs578T and MDA-MB-231, varying in COX-2 expression) as well as 10 human breast cancer specimens to examine the roles of COX-2 and prostaglandin E (EP) receptors in VEGF-C expression or secretion, and the relationship of COX-2 or VEGF-C expression to lymphangiogenesis. We found a strong correlation between COX-2 mRNA expression and VEGF-C expression or secretion levels in breast cancer cell lines and VEGF-C expression in breast cancer tissues. Expression of LYVE-1, a selective marker for lymphatic endothelium, was also positively correlated with COX-2 or VEGF-C expression in breast cancer tissues. Inhibition of VEGF-C expression and secretion in the presence of COX-1/2 or COX-2 inhibitors or following downregulation of COX-2 with COX-2 siRNA established a stimulatory role COX-2 in VEGF-C synthesis by breast cancer cells. EP1 as well as EP4 receptor antagonists inhibited VEGF-C production indicating the roles of EP1 and EP4 in VEGF-C upregulation by endogenous PGE2. Finally, VEGF-C secretion by MDA-MB-231 cells was inhibited in the presence of kinase inhibitors for Her-2/neu, Src and p38 MAPK, indicating a requirement of these kinases for VEGF-C synthesis. These results, for the first time, demonstrate a regulatory role of COX-2 in VEGF-C synthesis (and thereby lymphangiogenesis) in human breast cancer, which is mediated at least in part by EP1/EP4 receptors.
Figures





References
-
- Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG (2002) Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62: 1315–1320 - PubMed
-
- Breyer RM, Bagdassarian CK, Myers SA, Breyer MD (2001) Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41: 661–690 - PubMed
-
- Byeon JS, Jung HY, Lee YJ, Lee D, Lee GH, Myung SJ, Yang SK, Hong WS, Kim JH, Min YI, Kim JS (2004) Clinicopathological significance of vascular endothelial growth factor-C and cyclooxygenase-2 in esophageal squamous cell carcinoma. J Gastroenterol Hepatol 19: 648–654 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

