Dihydroceramide:sphinganine C-4-hydroxylation requires Des2 hydroxylase and the membrane form of cytochrome b5
- PMID: 16571104
- PMCID: PMC1513285
- DOI: 10.1042/BJ20051938
Dihydroceramide:sphinganine C-4-hydroxylation requires Des2 hydroxylase and the membrane form of cytochrome b5
Abstract
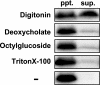
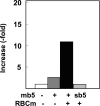
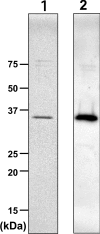
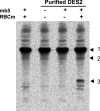
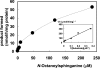
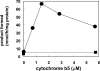
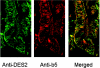
Des2 (degenerative spermatocyte 2) is a bifunctional enzyme that produces phytoceramide and ceramide from dihydroceramide. The molecular mechanism involved in C-4-hydroxylation has not been studied in detail. In the present paper, we report that C-4-hydroxylation requires an electron-transfer system that includes cytochrome b5 and that the hydroxylase activity is reconstituted in an in vitro assay with purified recombinant Des2. FLAG-tagged mouse Des2 was expressed in insect Sf9 cells and was purified by solubilization with digitonin and anti-FLAG antibody affinity column chromatography. The activity of dihydroceramide:sphinganine C-4-hydroxylase was reconstituted with the purified FLAG-Des2, mb5 (the membrane form of cytochrome b5) and bovine erythrocyte membrane. The apparent K(m) and V(max) of Des2 for the substrate N-octanoylsphinganine were 35 microM and 40 nmol x h(-1) x mg of protein(-1) respectively. The K(m) of the hydroxylase for mb5 was 0.8 microM. Interestingly, mb5 was not replaced with the soluble form of cytochrome b5, which lacks the C-terminal membrane-spanning domain. The erythrocyte membrane was separated into Triton X-100-soluble and -insoluble fractions, and the detergent-soluble fraction was replaced by the soluble or membrane form of b5R (NADH-cytochrome b5 reductase). The Triton-X-100-insoluble fraction contained trypsin-resistant factors. The Des2 protein is found in the endoplasmic reticulum and is assumed to have three membrane-spanning domains. The findings of the present study indicate that the hydroxylation requires complex formation between Des2 and mb5 via their membrane-spanning domains and electron transfer from NADH to the substrate via the reduction of mb5 by b5R.
Figures

References
-
- Smith W. L., Merrill A. H., Jr Sphingolipid metabolism and signaling minireview series. J. Biol. Chem. 2002;277:25841–25842. - PubMed
-
- Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. - PubMed
-
- Simons K., Vaz W. L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004;33:269–295. - PubMed
-
- Holthuis J. C., Pomorski T., Raggers R. J., Sprong H., Van Meer G. The organizing potential of sphingolipids in intracellular membrane transport. Physiol. Rev. 2001;81:1689–1723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

