Smad7 and protein phosphatase 1alpha are critical determinants in the duration of TGF-beta/ALK1 signaling in endothelial cells
- PMID: 16571110
- PMCID: PMC1479810
- DOI: 10.1186/1471-2121-7-16
Smad7 and protein phosphatase 1alpha are critical determinants in the duration of TGF-beta/ALK1 signaling in endothelial cells
Abstract
Background: In endothelial cells (EC), transforming growth factor-beta (TGF-beta) can bind to and transduce signals through ALK1 and ALK5. The TGF-beta/ALK5 and TGF-beta/ALK1 pathways have opposite effects on EC behaviour. Besides differential receptor binding, the duration of TGF-beta signaling is an important specificity determinant for signaling responses. TGF-beta/ALK1-induced Smad1/5 phosphorylation in ECs occurs transiently.
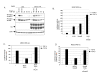
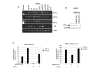
Results: The temporal activation of TGF-beta-induced Smad1/5 phosphorylation in ECs was found to be affected by de novo protein synthesis, and ALK1 and Smad5 expression levels determined signal strength of TGF-beta/ALK1 signaling pathway. Smad7 and protein phosphatase 1alpha (PP1alpha) mRNA expression levels were found to be specifically upregulated by TGF-beta/ALK1. Ectopic expression of Smad7 or PP1alpha potently inhibited TGF-beta/ALK1-induced Smad1/5 phosphorylation in ECs. Conversely, siRNA-mediated knockdown of Smad7 or PP1alpha enhanced TGF-beta/ALK1-induced signaling responses. PP1alpha interacted with ALK1 and this association was further potentiated by Smad7. Dephosphorylation of the ALK1, immunoprecipitated from cell lysates, was attenuated by a specific PP1 inhibitor.
Conclusion: Our results suggest that upon its induction by the TGF-beta/ALK1 pathway, Smad7 may recruit PP1alpha to ALK1, and thereby control TGF-beta/ALK1-induced Smad1/5 phosphorylation.
Figures


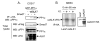
Similar articles
-
ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes.J Bone Miner Res. 2008 Jun;23(6):896-906. doi: 10.1359/jbmr.080209. J Bone Miner Res. 2008. PMID: 18333754
-
Differential TGF-{beta} signaling in retinal vascular cells: a role in diabetic retinopathy?Invest Ophthalmol Vis Sci. 2010 Apr;51(4):1857-65. doi: 10.1167/iovs.09-4181. Epub 2009 Dec 3. Invest Ophthalmol Vis Sci. 2010. PMID: 19959647
-
Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors.EMBO J. 2002 Apr 2;21(7):1743-53. doi: 10.1093/emboj/21.7.1743. EMBO J. 2002. PMID: 11927558 Free PMC article.
-
TGF-beta receptor function in the endothelium.Cardiovasc Res. 2005 Feb 15;65(3):599-608. doi: 10.1016/j.cardiores.2004.10.036. Cardiovasc Res. 2005. PMID: 15664386 Review.
-
Regulation of TGF-beta signaling by Smad7.Acta Biochim Biophys Sin (Shanghai). 2009 Apr;41(4):263-72. doi: 10.1093/abbs/gmp018. Acta Biochim Biophys Sin (Shanghai). 2009. PMID: 19352540 Free PMC article. Review.
Cited by
-
A rate equation approach to elucidate the kinetics and robustness of the TGF-beta pathway.Biophys J. 2006 Dec 15;91(12):4368-80. doi: 10.1529/biophysj.105.080408. Epub 2006 Sep 29. Biophys J. 2006. PMID: 17012329 Free PMC article.
-
Bone Morphogenetic Proteins in Vascular Homeostasis and Disease.Cold Spring Harb Perspect Biol. 2018 Feb 1;10(2):a031989. doi: 10.1101/cshperspect.a031989. Cold Spring Harb Perspect Biol. 2018. PMID: 28348038 Free PMC article. Review.
-
Combination of reverse and chemical genetic screens reveals angiogenesis inhibitors and targets.Chem Biol. 2009 Apr 24;16(4):432-41. doi: 10.1016/j.chembiol.2009.02.010. Chem Biol. 2009. PMID: 19389629 Free PMC article.
-
TGF-beta signal transduction in chronic kidney disease.Front Biosci (Landmark Ed). 2009 Jan 1;14(7):2448-65. doi: 10.2741/3389. Front Biosci (Landmark Ed). 2009. PMID: 19273211 Free PMC article. Review.
-
Regulation of TGF-beta signalling by protein phosphatases.Biochem J. 2010 Sep 1;430(2):191-8. doi: 10.1042/BJ20100427. Biochem J. 2010. PMID: 20704570 Free PMC article. Review.
References
-
- Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-β 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–31. doi: 10.1073/pnas.97.6.2626. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

