Experimental taphonomy shows the feasibility of fossil embryos
- PMID: 16571655
- PMCID: PMC1416897
- DOI: 10.1073/pnas.0601536103
Experimental taphonomy shows the feasibility of fossil embryos
Abstract
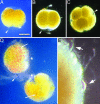
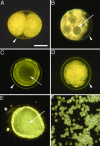
The recent discovery of apparent fossils of embryos contemporaneous with the earliest animal remains may provide vital insights into the metazoan radiation. However, although the putative fossil remains are similar to modern marine animal embryos or larvae, their simple geometric forms also resemble other organic and inorganic structures. The potential for fossilization of animals at such developmental stages and the taphonomic processes that might affect preservation before mineralization have not been examined. Here, we report experimental taphonomy of marine embryos and larvae similar in size and inferred cleavage mode to presumptive fossil embryos. Under conditions that prevent autolysis, embryos within the fertilization envelope can be preserved with good morphology for sufficiently long periods for mineralization to occur. The reported fossil record exhibits size bias, but we show that embryo size is unlikely to be a major factor in preservation. Under some conditions of death, fossilized remains will not accurately reflect the cell structure of the living organism. Although embryos within the fertilization envelope have high preservation potential, primary larvae have negligible preservation potential. Thus the paleo-embryological record may have strong biases on developmental stages preserved. Our data provide a predictive basis for interpreting the fossil record to unravel the evolution of ontogeny in the origin of metazoans.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

