RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex
- PMID: 16571679
- PMCID: PMC1474792
- DOI: 10.1091/mbc.e05-10-0973
RINT-1 regulates the localization and entry of ZW10 to the syntaxin 18 complex
Abstract
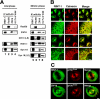
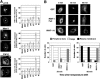
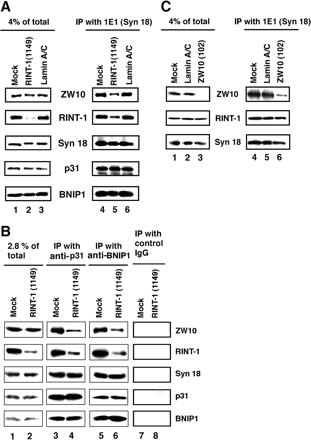
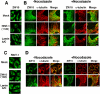
RINT-1 was first identified as a Rad50-interacting protein that participates in radiation-induced G2/M checkpoint control. We have recently reported that RINT-1, together with the dynamitin-interacting protein ZW10 and others, is associated with syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE involved in membrane trafficking between the ER and Golgi. To address the role of RINT-1 in membrane trafficking, we examined the effects of overexpression and knockdown of RINT-1 on Golgi morphology and protein transport from the ER. Overexpression of the N-terminal region of RINT-1, which is responsible for the interaction with ZW10, caused redistribution of ZW10. Concomitantly, ER-to-Golgi transport was blocked and the Golgi was dispersed. Knockdown of RINT-1 also disrupted membrane trafficking between the ER and Golgi. Notably, silencing of RINT-1 resulted in a reduction in the amount of ZW10 associated with syntaxin 18, concomitant with ZW10 redistribution. In contrast, no redistribution or release of RINT-1 from the syntaxin 18 complex was observed when ZW10 expression was reduced. These results taken together suggest that RINT-1 coordinates the localization and function of ZW10 by serving as a link between ZW10 and the SNARE complex comprising syntaxin 18.
Figures




References
-
- Andag U., Neumann T., Schmitt H. D. The coatomer-interacting protein Dsl1p is required for Golgi-to-endoplasmic reticulum retrieval in yeast. J. Biol. Chem. 2001;276:39150–39160. - PubMed
-
- Andag U., Schmitt H. D. Dsl1p, an essential component of the Golgi-endoplasmic reticulum retrieval system in yeast, uses the same sequence motif to interact with different subunits of the COPI vesicle coat. J. Biol. Chem. 2003;278:51722–51734. - PubMed
-
- Belgareh-Touze N., Corral-Debrinski M., Launhardt H., Galan J. M., Munder T., Le Panse S., Haguenauer-Tsapis R. Yeast functional analysis: identification of two essential genes involved in ER to Golgi trafficking. Traffic. 2003;4:607–617. - PubMed
-
- Bonifacino J. S., Glick B. S. The mechanism of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

