Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway
- PMID: 16571730
- PMCID: PMC3668334
- DOI: 10.1074/jbc.M512953200
Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway
Abstract
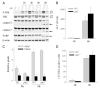
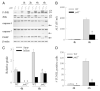
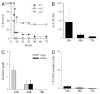
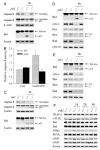
In vitro studies of hepatocytes have implicated over-activation of c-Jun N-terminal kinase (JNK) signaling as a mechanism of tumor necrosis factor-alpha (TNF)-induced apoptosis. However, the functional significance of JNK activation and the role of specific JNK isoforms in TNF-induced hepatic apoptosis in vivo remain unclear. JNK1 and JNK2 function was, therefore, investigated in the TNF-dependent, galactosamine/lipopolysaccharide (GalN/LPS) model of liver injury. The toxin GalN converted LPS-induced JNK signaling from a transient to prolonged activation. Liver injury and mortality from GalN/LPS was equivalent in wild-type and jnk1-/- mice but markedly decreased in jnk2-/- mice. This effect was not secondary to down-regulation of TNF receptor 1 expression or TNF production. In the absence of jnk2, the caspase-dependent, TNF death pathway was blocked, as reflected by the failure of caspase-3 and -7 and poly(ADP-ribose) polymerase cleavage to occur. JNK2 was critical for activation of the mitochondrial death pathway, as in jnk2-/- mice Bid cleavage and mitochondrial translocation and cytochrome c release were markedly decreased. This effect was secondary to the failure of jnk2-/- mice to activate caspase-8. Liver injury and caspase activation were similarly decreased in jnk2 null mice after GalN/TNF treatment. Ablation of jnk2 did not inhibit GalN/LPS-induced c-Jun kinase activity, although activity was completely blocked in jnk1-/- mice. Toxic liver injury is, therefore, associated with JNK over-activation and mediated by JNK2 promotion of caspase-8 activation and the TNF mitochondrial death pathway through a mechanism independent of c-Jun kinase activity.
Figures




Similar articles
-
The role of JNK2 in toxic liver injury.J Hepatol. 2006 Nov;45(5):762-4. doi: 10.1016/j.jhep.2006.08.004. Epub 2006 Sep 1. J Hepatol. 2006. PMID: 16979778
-
Hepatotoxicity mediated by pyrazole (cytochrome P450 2E1) plus tumor necrosis factor alpha treatment occurs in c-Jun N-terminal kinase 2-/- but not in c-Jun N-terminal kinase 1-/- mice.Hepatology. 2011 Nov;54(5):1753-66. doi: 10.1002/hep.24540. Hepatology. 2011. PMID: 21748763 Free PMC article.
-
Antiapoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis.Gastroenterology. 2009 Apr;136(4):1423-34. doi: 10.1053/j.gastro.2008.12.064. Epub 2009 Jan 6. Gastroenterology. 2009. PMID: 19249395
-
Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury.Gastroenterology. 2016 Apr;150(4):968-81. doi: 10.1053/j.gastro.2015.12.019. Epub 2015 Dec 19. Gastroenterology. 2016. PMID: 26708719 Free PMC article.
-
c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis.Mol Cell Biol. 2004 Dec;24(24):10844-56. doi: 10.1128/MCB.24.24.10844-10856.2004. Mol Cell Biol. 2004. PMID: 15572687 Free PMC article.
Cited by
-
A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches.Gastroenterology. 2012 Aug;143(2):307-20. doi: 10.1053/j.gastro.2012.06.004. Epub 2012 Jun 13. Gastroenterology. 2012. PMID: 22705006 Free PMC article. Review.
-
A nanoconjugate Apaf-1 inhibitor protects mesothelial cells from cytokine-induced injury.PLoS One. 2009 Aug 13;4(8):e6634. doi: 10.1371/journal.pone.0006634. PLoS One. 2009. PMID: 19675677 Free PMC article.
-
cAMP-guanine exchange factor protection from bile acid-induced hepatocyte apoptosis involves glycogen synthase kinase regulation of c-Jun NH2-terminal kinase.Am J Physiol Gastrointest Liver Physiol. 2011 Aug;301(2):G385-400. doi: 10.1152/ajpgi.00430.2010. Epub 2011 May 5. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21546580 Free PMC article.
-
Inhibition of the mitochondrial permeability transition by cyclosporin A prevents pyrazole plus lipopolysaccharide-induced liver injury in mice.Free Radic Biol Med. 2009 Feb 1;46(3):406-13. doi: 10.1016/j.freeradbiomed.2008.10.037. Epub 2008 Oct 31. Free Radic Biol Med. 2009. PMID: 19026739 Free PMC article.
-
Role of C-Jun N-Terminal Kinases on a Stressed Epithelium: Time for Testing Isoform Specificity.Biology (Basel). 2025 Jun 3;14(6):649. doi: 10.3390/biology14060649. Biology (Basel). 2025. PMID: 40563900 Free PMC article. Review.
References
-
- Czaja MJ, Xu J, Alt E. Gastroenterology. 1995;108:1849–1854. - PubMed
-
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Gastroenterology. 1999;117:942–952. - PubMed
-
- Teoh N, Field J, Sutton J, Farrell G. Hepatology. 2004;39:412–421. - PubMed
-
- Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Hepatology. 2001;33:902–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

