B-ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus
- PMID: 16571754
- PMCID: PMC6673859
- DOI: 10.1523/JNEUROSCI.4338-05.2006
B-ephrin reverse signaling is required for NMDA-independent long-term potentiation of mossy fibers in the hippocampus
Abstract
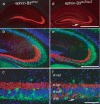
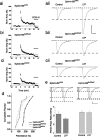
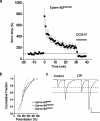
The mossy fiber to CA3 pyramidal neuron synapse in the hippocampus displays an atypical form of NMDA receptor-independent long-term potentiation (LTP). Plasticity at this synapse is expressed in the presynaptic terminal as an elevated probability of neurotransmitter release. However, evidence indicates that postsynaptic mechanisms and trans-synaptic signaling through an association between postsynaptic EphB receptors and presynaptic B-ephrins are necessary for the induction of LTP. Here we show that ephrin-B3 protein is highly expressed in mossy fiber axons and terminals. There are specific deficits in mossy fiber LTP in mice in which the cytoplasmic C-terminal signaling domain of the ephrin-B3 protein is replaced with beta-galactosidase. These deficits are not observed in ephrin-B3 null mutant mice because of functional redundancy by virtue of other B-ephrins. These results indicate that B-ephrin reverse signaling into the presynaptic mossy fiber bouton is required for the induction of NMDA receptor-independent LTP at this synapse.
Figures




References
-
- Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG (1998). Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene 16:471–480. - PubMed
-
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA (1997). Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388:590–593. - PubMed
-
- Castillo PE, Schoch S, Schmitz F, Sudhof TC, Malenka RC (2002). RIM1alpha is required for presynaptic long-term potentiation. Nature 415:327–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
