Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release
- PMID: 16571755
- PMCID: PMC6673869
- DOI: 10.1523/JNEUROSCI.4171-05.2006
Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release
Abstract
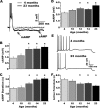
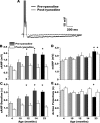
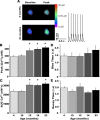
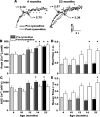
Age-dependent changes in multiple Ca2+-related electrophysiological processes in the hippocampus appear to be consistent biomarkers of aging, and several also correlate with cognitive decline. These findings have led to the hypothesis that a common mechanism of Ca2+ dyshomeostasis underlies aspects of aging-dependent brain impairment. However, some key predictions of this view remain untested, including that multiple Ca2+-related biomarkers should emerge concurrently during aging and their onset should also precede/coincide with initial signs of cognitive decline. Moreover, blocking a putative common source of dysregulated Ca2+ should eliminate aging differences. Here, we tested these predictions using combined electrophysiological, imaging, and pharmacological approaches in CA1 neurons to determine the ages of onset (across 4-, 10-, 12-, 14-, and 23-month-old F344 rats) of several established biomarkers, including the increases in the slow afterhyperpolarization, spike accommodation, and [Ca2+]i rise during repetitive synaptic stimulation. In addition, we tested the hypothesis that altered Ca2+-induced Ca2+ release (CICR) from ryanodine receptors, which can be triggered by L-type Ca2+ channels, provides a common source of dysregulated Ca2+ in aging. Results showed that multiple aging biomarkers were first detectable at about the same age (12 months of age; approximately midlife), sufficiently early to influence initial cognitive decline. Furthermore, selectively blocking CICR with ryanodine slowed the Ca2+ rise during synaptic stimulation more in aged rat neurons and, notably, reduced or eliminated aging differences in the biomarkers. Thus, this study provides the first evidence that altered CICR plays a role in driving the early and simultaneous emergence in hippocampus of multiple Ca2+-related biomarkers of aging.
Figures
References
-
- Aitken DH, Meaney MJ (1989). Temporally graded, age-related impairments in spatial memory in the rat. Neurobiol Aging 10:273–276. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER (1999). Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA 96:5280–5285. - PMC - PubMed
-
- Barnes CA (1994). Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci 17:13–18. - PubMed
-
- Barnes CA, Rao G, Shen J (1997). Age-related decrease in the N-methyl-d-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging 18:445–452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
