Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling
- PMID: 16571757
- PMCID: PMC6673845
- DOI: 10.1523/JNEUROSCI.3792-05.2006
Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling
Abstract
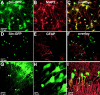
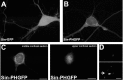
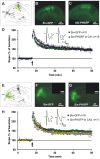
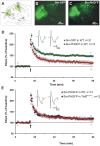
Neurotrophins have been shown to play a critical role in activity-dependent synaptic plasticity such as long-term potentiation (LTP) in the hippocampus. Although the role of brain-derived neurotrophic factor (BDNF) and its tyrosine kinase receptor [tyrosine receptor kinase B (TrkB)] is well documented, it still remains unresolved whether presynaptic or postsynaptic activation of TrkB is involved in the induction of LTP. To address this question, we locally and specifically interfered with a downstream target of the TrkB receptor, phospholipase Cgamma (PLCgamma). We prevented PLCgamma signaling by overexpression of the PLCgamma pleckstrin homology (PH) domain with a Sindbis virus vector. The isolated PH domain has an inhibitory effect and thereby blocks endogenous PLCgamma signaling and consequently also IP3 production. Surprisingly, concurrent presynaptic and postsynaptic blockade of PLCgamma signaling was required to reduce LTP to levels comparable with those in TrkB and BDNF knock-out mice. Blockade of presynaptic or postsynaptic signaling alone did not result in a significant reduction of LTP.
Figures
Similar articles
-
Acute and chronic interference with BDNF/TrkB-signaling impair LTP selectively at mossy fiber synapses in the CA3 region of mouse hippocampus.Neuropharmacology. 2013 Aug;71:247-54. doi: 10.1016/j.neuropharm.2013.03.041. Epub 2013 Apr 12. Neuropharmacology. 2013. PMID: 23587649
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
Genetic Dissection of Presynaptic and Postsynaptic BDNF-TrkB Signaling in Synaptic Efficacy of CA3-CA1 Synapses.Cell Rep. 2018 Aug 7;24(6):1550-1561. doi: 10.1016/j.celrep.2018.07.020. Cell Rep. 2018. PMID: 30089265 Free PMC article.
-
Blockade of BDNF signaling turns chemically-induced long-term potentiation into long-term depression.Hippocampus. 2013 Oct;23(10):879-89. doi: 10.1002/hipo.22144. Epub 2013 Jun 26. Hippocampus. 2013. PMID: 23674394
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
Cited by
-
Endogenous brain-derived neurotrophic factor triggers fast calcium transients at synapses in developing dendrites.J Neurosci. 2007 Jan 31;27(5):1097-105. doi: 10.1523/JNEUROSCI.3590-06.2007. J Neurosci. 2007. PMID: 17267564 Free PMC article.
-
mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch.Neuron. 2011 Apr 28;70(2):339-51. doi: 10.1016/j.neuron.2011.02.045. Neuron. 2011. PMID: 21521618 Free PMC article.
-
BDNF regulates the expression and distribution of vesicular glutamate transporters in cultured hippocampal neurons.PLoS One. 2013;8(1):e53793. doi: 10.1371/journal.pone.0053793. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326507 Free PMC article.
-
Inositol 1,4,5-trisphosphate 3-kinase A is a novel microtubule-associated protein: PKA-dependent phosphoregulation of microtubule binding affinity.J Biol Chem. 2012 May 4;287(19):15981-95. doi: 10.1074/jbc.M112.344101. Epub 2012 Mar 2. J Biol Chem. 2012. PMID: 22389500 Free PMC article.
-
Early phase of plasticity-related gene regulation and SRF dependent transcription in the hippocampus.PLoS One. 2013 Jul 23;8(7):e68078. doi: 10.1371/journal.pone.0068078. Print 2013. PLoS One. 2013. PMID: 23935853 Free PMC article.
References
-
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S (1993). Modified hippocampal long-term potentiation in PKCgamma-mutant mice. Cell 75:1253–1262. - PubMed
-
- Behnisch T, Reymann KG (1995). Thapsigargin blocks long-term potentiation induced by weak, but not strong tetanisation in rat hippocampal CA1 neurons. Neurosci Lett 192:185–188. - PubMed
-
- Blum R, Kafitz KW, Konnerth A (2002). Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature 419:687–693. - PubMed
-
- Brewer GJ, Cotman CW (1989). Survival and growth of hippocampal neurons in defined medium at low density: advantages of a sandwich culture technique or low oxygen. Brain Res 494:65–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
