Nuclear factor kappaB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss
- PMID: 16571762
- PMCID: PMC2897814
- DOI: 10.1523/JNEUROSCI.2488-05.2006
Nuclear factor kappaB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss
Abstract
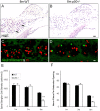
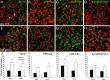
Degeneration of the spiral ganglion neurons (SGNs) of the auditory nerve occurs with age and in response to acoustic injury. Histopathological observations suggest that the neural degeneration often begins with an excitotoxic process affecting the afferent dendrites under the inner hair cells (IHCs), however, little is known about the sequence of cellular or molecular events mediating this excitotoxicity. Nuclear factor kappaB (NFkappaB) is a transcription factor involved in regulating inflammatory responses and apoptosis in many cell types. NFkappaB is also associated with intracellular calcium regulation, an important factor in neuronal excitotoxicity. Here, we provide evidence that NFkappaB can play a central role in the degeneration of SGNs. Mice lacking the p50 subunit of NFkappaB (p50(-/-) mice) showed an accelerated hearing loss with age that was highly associated with an exacerbated excitotoxic-like damage in afferent dendrites under IHCs and an accelerated loss of SGNs. Also, as evidenced by immunostaining intensity, calcium-buffering proteins were significantly elevated in SGNs of the p50(-/-) mice. Finally, the knock-out mice exhibited an increased sensitivity to low-level noise exposure. The accelerated hearing loss and neural degeneration with age in the p50(-/-) mice occurred in the absence of concomitant hair cell loss and decline of the endocochlear potential. These results indicate that NFkappaB activity plays an important role in protecting the primary auditory neurons from excitotoxic damage and age-related degeneration. A possible mechanism underlying this protection is that the NFkappaB activity may help to maintain calcium homeostasis in SGNs.
Figures





Similar articles
-
[Role of glutamate receptors in the spiral ganglion neuron damage induced by acoustic noise].Sheng Li Xue Bao. 2007 Feb 25;59(1):103-10. Sheng Li Xue Bao. 2007. PMID: 17294049 Chinese.
-
Genetic disruption of fractalkine signaling leads to enhanced loss of cochlear afferents following ototoxic or acoustic injury.J Comp Neurol. 2018 Apr 1;526(5):824-835. doi: 10.1002/cne.24369. Epub 2017 Dec 17. J Comp Neurol. 2018. PMID: 29218724 Free PMC article.
-
Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss.Hear Res. 2015 Dec;330(Pt B):191-9. doi: 10.1016/j.heares.2015.02.009. Epub 2015 Mar 11. Hear Res. 2015. PMID: 25769437 Free PMC article.
-
Age-related loss of spiral ganglion neurons.Hear Res. 2010 Jun 1;264(1-2):93-7. doi: 10.1016/j.heares.2009.10.009. Epub 2009 Oct 23. Hear Res. 2010. PMID: 19854255 Free PMC article. Review.
-
Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms.Hear Res. 2017 Jun;349:138-147. doi: 10.1016/j.heares.2017.01.003. Epub 2017 Jan 10. Hear Res. 2017. PMID: 28087419 Free PMC article. Review.
Cited by
-
ROR1 is essential for proper innervation of auditory hair cells and hearing in humans and mice.Proc Natl Acad Sci U S A. 2016 May 24;113(21):5993-8. doi: 10.1073/pnas.1522512113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162350 Free PMC article.
-
Manganese-mediated acceleration of age-related hearing loss in mice.Sci Rep. 2016 Nov 8;6:36306. doi: 10.1038/srep36306. Sci Rep. 2016. PMID: 27824154 Free PMC article.
-
Recent findings and emerging questions in cochlear noise injury.Hear Res. 2008 Nov;245(1-2):5-17. doi: 10.1016/j.heares.2008.08.007. Epub 2008 Aug 29. Hear Res. 2008. PMID: 18790034 Free PMC article. Review. No abstract available.
-
Application of Mouse Models to Research in Hearing and Balance.J Assoc Res Otolaryngol. 2016 Dec;17(6):493-523. doi: 10.1007/s10162-016-0589-1. Epub 2016 Oct 17. J Assoc Res Otolaryngol. 2016. PMID: 27752925 Free PMC article. Review.
-
Variation in genes related to cochlear biology is strongly associated with adult-onset deafness in border collies.PLoS Genet. 2012 Sep;8(9):e1002898. doi: 10.1371/journal.pgen.1002898. Epub 2012 Sep 13. PLoS Genet. 2012. PMID: 23028339 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
