Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia
- PMID: 16571765
- PMCID: PMC6673853
- DOI: 10.1523/JNEUROSCI.5050-05.2006
Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia
Abstract
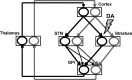
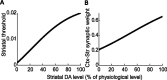
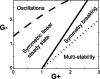
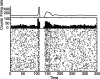
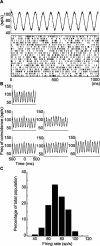
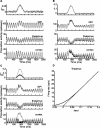
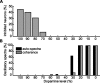
Experiments performed in normal animals suggest that the basal ganglia (BG) are crucial in motor program selection. BG are also involved in movement disorders. In particular, BG neuronal activity in parkinsonian animals and patients is more oscillatory and more synchronous than in normal individuals. We propose a new model for the function and dysfunction of the motor part of BG. We hypothesize that the striatum, the subthalamic nucleus, the internal pallidum (GPi), the thalamus, and the cortex are involved in closed feedback loops. The direct (cortex-striatum-GPi-thalamus-cortex) and the hyperdirect loops (cortex-subthalamic nucleus-GPi-thalamus-cortex), which have different polarities, play a key role in the model. We show that the competition between these two loops provides the BG-cortex system with the ability to perform motor program selection. Under the assumption that dopamine potentiates corticostriatal synaptic transmission, we demonstrate that, in our model, moderate dopamine depletion leads to a complete loss of action selection ability. High depletion can lead to synchronous oscillations. These modifications of the network dynamical state stem from an imbalance between the feedback in the direct and hyperdirect loops when dopamine is depleted. Our model predicts that the loss of selection ability occurs before the appearance of oscillations, suggesting that Parkinson's disease motor impairments are not directly related to abnormal oscillatory activity. Another major prediction of our model is that synchronous oscillations driven by the hyperdirect loop appear in BG after inactivation of the striatum.
Figures






Comment in
-
Emergent basal ganglia pathology within computational models.J Neurosci. 2006 Jul 12;26(28):7317-8. doi: 10.1523/jneurosci.2255-06.2006. J Neurosci. 2006. PMID: 16838428 Free PMC article. No abstract available.
Similar articles
-
Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease.Mov Disord. 2008;23 Suppl 3:S548-59. doi: 10.1002/mds.22062. Mov Disord. 2008. PMID: 18781672 Review.
-
Untangling Basal Ganglia Network Dynamics and Function: Role of Dopamine Depletion and Inhibition Investigated in a Spiking Network Model.eNeuro. 2017 Jan 12;3(6):ENEURO.0156-16.2016. doi: 10.1523/ENEURO.0156-16.2016. eCollection 2016 Nov-Dec. eNeuro. 2017. PMID: 28101525 Free PMC article.
-
Mean-field modeling of the basal ganglia-thalamocortical system. II Dynamics of parkinsonian oscillations.J Theor Biol. 2009 Apr 21;257(4):664-88. doi: 10.1016/j.jtbi.2008.12.013. Epub 2008 Dec 24. J Theor Biol. 2009. PMID: 19154745
-
Basal ganglia oscillations and pathophysiology of movement disorders.Curr Opin Neurobiol. 2006 Dec;16(6):629-37. doi: 10.1016/j.conb.2006.10.002. Epub 2006 Nov 3. Curr Opin Neurobiol. 2006. PMID: 17084615 Review.
-
Interaction of oscillations, and their suppression via deep brain stimulation, in a model of the cortico-basal ganglia network.IEEE Trans Neural Syst Rehabil Eng. 2013 Mar;21(2):244-53. doi: 10.1109/TNSRE.2013.2241791. IEEE Trans Neural Syst Rehabil Eng. 2013. PMID: 23476006
Cited by
-
Spike-Timing-Dependent Plasticity Mediated by Dopamine and its Role in Parkinson's Disease Pathophysiology.Front Netw Physiol. 2022 Mar 4;2:817524. doi: 10.3389/fnetp.2022.817524. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926058 Free PMC article. Review.
-
A physiologically plausible model of action selection and oscillatory activity in the basal ganglia.J Neurosci. 2006 Dec 13;26(50):12921-42. doi: 10.1523/JNEUROSCI.3486-06.2006. J Neurosci. 2006. PMID: 17167083 Free PMC article.
-
Thinking Outside the Box (and Arrow): Current Themes in Striatal Dysfunction in Movement Disorders.Neuroscientist. 2019 Aug;25(4):359-379. doi: 10.1177/1073858418807887. Epub 2018 Oct 31. Neuroscientist. 2019. PMID: 30379121 Free PMC article. Review.
-
Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models.Front Comput Neurosci. 2013 Dec 6;7:174. doi: 10.3389/fncom.2013.00174. Front Comput Neurosci. 2013. PMID: 24367325 Free PMC article. Review.
-
Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson's disease.PLoS One. 2014 Jan 22;9(1):e86496. doi: 10.1371/journal.pone.0086496. eCollection 2014. PLoS One. 2014. PMID: 24466120 Free PMC article.
References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A (2000). Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3:226–230. - PubMed
-
- Albin RL, Young AB, Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375. - PubMed
-
- Alexander GE, DeLong MR, Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. - PubMed
-
- Apicella P, Ljungberg T, Scarnati E, Schultz W (1991). Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85:491–500. - PubMed
-
- Baker SN, Gerstein GL (2001). Determination of response latency and its application to normalization of cross-correlation measures. Neural Comput 13:1351–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
