Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM
- PMID: 16571794
- PMCID: PMC1440471
- DOI: 10.1128/JVI.80.8.3773-3780.2006
Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM
Abstract
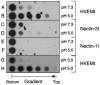
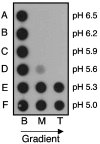
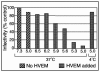
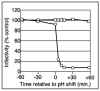
Using a liposome-binding assay, we investigated the requirements for activation of herpes simplex virus (HSV) into a state capable of membrane interaction. Virions were mixed with liposomes along with the ectodomain of one of three gD receptors (HVEMt, nectin-1t, or nectin-2t) and incubated under different pH and temperature conditions. Virions failed to associate with liposomes in the presence of nectin-1 or nectin-2 at any temperature or pH tested. In contrast, HVEMt triggered association of HSV with liposomes at pH 5.3 or 5.0 when incubated at 37 degrees C, suggesting that HVEM binding and mildly acidic pH at a physiological temperature provide coactivation signals, allowing virus association with membranes. Virions incubated with HVEMt at 37 degrees C without liposomes rapidly lost infectivity upon exposure to pH 5.0, suggesting that these conditions lead to irreversible virus inactivation in the absence of target membranes. Consistent with the idea that soluble receptor molecules provide a trigger for HSV entry, HVEMt promoted virus entry into receptor-deficient CHO K1 cells. However, in B78H1 cells, HVEMt promoted virus entry with markedly lower efficiency. Interestingly, HSV entry into receptor-bearing CHO K1 cells has been shown to proceed via a pH-dependent manner, whereas HSV entry into receptor-bearing B78H1 cells is pH independent. Based on these observations, we propose that the changes triggered by HVEM and mildly acidic pH that allow liposome association are similar or identical to changes that occur during pH-dependent HSV entry.
Figures



Similar articles
-
Nectin-2-mediated entry of a syncytial strain of herpes simplex virus via pH-independent fusion with the plasma membrane of Chinese hamster ovary cells.Virol J. 2006 Dec 27;3:105. doi: 10.1186/1743-422X-3-105. Virol J. 2006. PMID: 17192179 Free PMC article.
-
Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM.J Virol. 1998 May;72(5):3595-601. doi: 10.1128/JVI.72.5.3595-3601.1998. J Virol. 1998. PMID: 9557640 Free PMC article.
-
Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1.J Virol. 2003 Sep;77(17):9221-31. doi: 10.1128/jvi.77.17.9221-9231.2003. J Virol. 2003. PMID: 12915538 Free PMC article.
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
-
The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells.Rev Med Virol. 2000 Sep-Oct;10(5):305-19. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. Rev Med Virol. 2000. PMID: 11015742 Review.
Cited by
-
Induction of conformational changes at the N-terminus of herpes simplex virus glycoprotein D upon binding to HVEM and nectin-1.Virology. 2014 Jan 5;448:185-95. doi: 10.1016/j.virol.2013.10.019. Epub 2013 Oct 29. Virology. 2014. PMID: 24314649 Free PMC article.
-
Mildly Acidic pH Triggers an Irreversible Conformational Change in the Fusion Domain of Herpes Simplex Virus 1 Glycoprotein B and Inactivation of Viral Entry.J Virol. 2017 Feb 14;91(5):e02123-16. doi: 10.1128/JVI.02123-16. Print 2017 Mar 1. J Virol. 2017. PMID: 28003487 Free PMC article.
-
Role of the UL45 protein in herpes simplex virus entry via low pH-dependent endocytosis and its relationship to the conformation and function of glycoprotein B.Virus Res. 2010 Apr;149(1):115-8. doi: 10.1016/j.virusres.2010.01.004. Epub 2010 Jan 18. Virus Res. 2010. PMID: 20080138 Free PMC article.
-
Reversible conformational change in herpes simplex virus glycoprotein B with fusion-from-without activity is triggered by mildly acidic pH.Virol J. 2010 Dec 1;7:352. doi: 10.1186/1743-422X-7-352. Virol J. 2010. PMID: 21122119 Free PMC article.
-
Herpes simplex virus 1 glycoprotein C promotes virus penetration from endosomes during entry, independent of interaction with heparan sulfate.Front Microbiol. 2025 Apr 9;16:1549349. doi: 10.3389/fmicb.2025.1549349. eCollection 2025. Front Microbiol. 2025. PMID: 40270821 Free PMC article.
References
-
- Bullough, P. A., F. M. Hughson, J. K. Skehel, and D. C. Wiley. 2002. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. - PubMed
-
- Cohen, G. H., V. J. Isola, J. Kuhns, P. W. Berman, and R. J. Eisenberg. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J. Virol. 60:157-166. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

