Antibody production and in vitro behavior of CD27-defined B-cell subsets: persistent hepatitis C virus infection changes the rules
- PMID: 16571809
- PMCID: PMC1440441
- DOI: 10.1128/JVI.80.8.3923-3934.2006
Antibody production and in vitro behavior of CD27-defined B-cell subsets: persistent hepatitis C virus infection changes the rules
Abstract
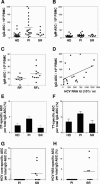
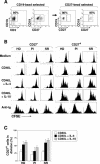
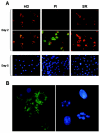
There is growing interest in the tendency of B cells to change their functional program in response to overwhelming antigen loading, perhaps by regulating specific parameters, such as efficiency of activation, proliferation rate, differentiation to antibody-secreting cells (ASC), and rate of cell death in culture. We show that individuals persistently infected with hepatitis C virus (HCV) carry high levels of circulating immunoglobulin G (IgG) and IgG-secreting cells (IgG-ASC). Thus, generalized polyclonal activation of B-cell functions may be supposed. While IgGs include virus-related and unrelated antibodies, IgG-ASC do not include HCV-specific plasma cells. Despite signs of widespread activation, B cells do not accumulate and memory B cells seem to be reduced in the blood of HCV-infected individuals. This apparent discrepancy may reflect the unconventional activation kinetics and functional responsiveness of the CD27+ B-cell subset in vitro. Following stimulation with T-cell-derived signals in the absence of B-cell receptor (BCR) engagement, CD27+ B cells do not expand but rapidly differentiate to secrete Ig and then undergo apoptosis. We propose that their enhanced sensitivity to BCR-independent noncognate T-cell help maintains a constant level of nonspecific serum antibodies and ASC and serves as a backup mechanism of feedback inhibition to prevent exaggerated B-cell responses that could be the cause of significant immunopathology.
Figures




Similar articles
-
Clonal CD27+ CD19+ B cell expansion through inhibition of FC gammaIIR in HCV(+) cryoglobulinemic patients.Ann N Y Acad Sci. 2009 Sep;1173:326-33. doi: 10.1111/j.1749-6632.2009.04664.x. Ann N Y Acad Sci. 2009. PMID: 19758169
-
Analysis of B-lymphocyte differentiation in patients infected with hepatitis C virus.J Med Virol. 2004 Apr;72(4):566-74. doi: 10.1002/jmv.20039. J Med Virol. 2004. PMID: 14981759
-
Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections.J Hepatol. 2011 Jul;55(1):53-60. doi: 10.1016/j.jhep.2010.10.016. Epub 2010 Nov 23. J Hepatol. 2011. PMID: 21145853
-
IgM and IgG but not cytokine secretion is restricted to the CD27+ B lymphocyte subset.J Immunol. 1992 Jun 15;148(12):3700-5. J Immunol. 1992. PMID: 1318333
-
Expression of CD45RB and CD27 identifies subsets of CD4+ memory T cells with different capacities to induce B cell differentiation.J Immunol. 1995 Jul 1;155(1):149-62. J Immunol. 1995. PMID: 7541412
Cited by
-
Molecular signatures of hepatitis C virus (HCV)-induced type II mixed cryoglobulinemia (MCII).Viruses. 2012 Nov 8;4(11):2924-44. doi: 10.3390/v4112924. Viruses. 2012. PMID: 23202510 Free PMC article. Review.
-
Remodeling of B-Cell Subsets in Blood during Pegylated IFNα-2a Therapy in Patients with Chronic Hepatitis B Infection.PLoS One. 2016 Jun 9;11(6):e0156200. doi: 10.1371/journal.pone.0156200. eCollection 2016. PLoS One. 2016. PMID: 27281019 Free PMC article. Clinical Trial.
-
CD45RB Glycosylation and Ig Isotype Define Maturation of Functionally Distinct B Cell Subsets in Human Peripheral Blood.Front Immunol. 2022 Apr 28;13:891316. doi: 10.3389/fimmu.2022.891316. eCollection 2022. Front Immunol. 2022. PMID: 35572548 Free PMC article.
-
Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence.J Clin Invest. 2009 Jul;119(7):1745-54. doi: 10.1172/JCI39133. Epub 2009 Jul 1. J Clin Invest. 2009. PMID: 19587449 Free PMC article. Review.
-
Type-I Interferon Responses: From Friend to Foe in the Battle against Chronic Viral Infection.Front Immunol. 2016 Dec 19;7:609. doi: 10.3389/fimmu.2016.00609. eCollection 2016. Front Immunol. 2016. PMID: 28066419 Free PMC article. Review.
References
-
- Agematsu, K., H. Nagumo, F. C. Yang, T. Nakazawa, K. Fukushima, S. Ito, K. Sugita, T. Mori, T. Kobata, C. Morimoto, and A. Komiyama. 1997. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27:2073-2079. - PubMed
-
- Armitage, R. J., B. M. Macduff, J. Eisenman, R. Paxton, and K. H. Grabstein. 1995. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 154:483-490. - PubMed
-
- Armitage, R. J., B. M. Macduff, M. K. Spriggs, and W. C. Fanslow. 1993. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J. Immunol. 150:3671-3680. - PubMed
-
- Arpin, C., J. Dechanet, C. Van Kooten, P. Merville, G. Grouard, F. Briere, J. Banchereau, and Y. J. Liu. 1995. Generation of memory B cells and plasma cells in vitro. Science 268:720-722. - PubMed
-
- Avery, D. T., J. I. Ellyard, F. Mackay, L. M. Corcoran, P. D. Hodgkin, and S. G. Tangye. 2005. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J. Immunol. 174:4034-4042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

