Rotavirus spike protein VP4 binds to and remodels actin bundles of the epithelial brush border into actin bodies
- PMID: 16571811
- PMCID: PMC1440440
- DOI: 10.1128/JVI.80.8.3947-3956.2006
Rotavirus spike protein VP4 binds to and remodels actin bundles of the epithelial brush border into actin bodies
Abstract
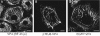
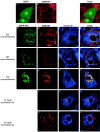
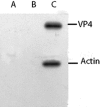
We demonstrate here that VP4, a rotaviral protein, is able to specifically bind to bundled actin microfilaments that are subsequently profoundly remodeled into actin bodies. These cytoplasmic actin bodies do not localize within identified intracellular compartments. VP4-induced actin remodeling is similar to cytochalasin D effects with kinetics compatible with that of rotavirus infection. Actin bundles' remodeling occurs both in infected and in VP4-transfected cells and in various cell lines, indicating that this is a general property of the viral protein itself. Interestingly, in intestinal epithelial cells, which represent the natural target of rotavirus, VP4 is addressed to the apical membrane where it binds specifically to brush border actin bundles and elicits its remodeling, whereas cytochalasin D impaired all the filamentous actin. These observations indicate that these original properties of VP4 likely explain the previously described brush border alterations that follow rotavirus infection of enterocytes and may also participate to the mechanism of rotavirus final assembly.
Figures





Similar articles
-
Heterogeneity of Raft-type membrane microdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells.J Virol. 2007 Feb;81(4):1610-8. doi: 10.1128/JVI.01433-06. Epub 2006 Nov 29. J Virol. 2007. PMID: 17135322 Free PMC article.
-
Role for actin in the polarized release of rotavirus.J Virol. 2007 May;81(9):4892-4. doi: 10.1128/JVI.02698-06. Epub 2007 Feb 14. J Virol. 2007. PMID: 17301135 Free PMC article.
-
The C Terminus of Rotavirus VP4 Protein Contains an Actin Binding Domain Which Requires Cooperation with the Coiled-Coil Domain for Actin Remodeling.J Virol. 2018 Dec 10;93(1):e01598-18. doi: 10.1128/JVI.01598-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30333172 Free PMC article.
-
Rotavirus assembly: an alternative model that utilizes an atypical trafficking pathway.Curr Top Microbiol Immunol. 2006;309:245-61. doi: 10.1007/3-540-30773-7_9. Curr Top Microbiol Immunol. 2006. PMID: 16909902 Free PMC article. Review.
-
Plasticity of the brush border - the yin and yang of intestinal homeostasis.Nat Rev Gastroenterol Hepatol. 2016 Mar;13(3):161-74. doi: 10.1038/nrgastro.2016.5. Epub 2016 Feb 3. Nat Rev Gastroenterol Hepatol. 2016. PMID: 26837713 Review.
Cited by
-
Rotavirus-Induced Early Activation of the RhoA/ROCK/MLC Signaling Pathway Mediates the Disruption of Tight Junctions in Polarized MDCK Cells.Sci Rep. 2018 Sep 17;8(1):13931. doi: 10.1038/s41598-018-32352-y. Sci Rep. 2018. PMID: 30224682 Free PMC article.
-
Expression of rotavirus NSP4 alters the actin network organization through the actin remodeling protein cofilin.J Virol. 2007 Apr;81(7):3545-53. doi: 10.1128/JVI.01080-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229686 Free PMC article.
-
Rotavirus NSP2: A Master Orchestrator of Early Viral Particle Assembly.Viruses. 2024 May 21;16(6):814. doi: 10.3390/v16060814. Viruses. 2024. PMID: 38932107 Free PMC article. Review.
-
Actin cytoskeleton remodeling primes RIG-I-like receptor activation.Cell. 2022 Sep 15;185(19):3588-3602.e21. doi: 10.1016/j.cell.2022.08.011. Cell. 2022. PMID: 36113429 Free PMC article.
-
Heterogeneity of Raft-type membrane microdomains associated with VP4, the rotavirus spike protein, in Caco-2 and MA 104 cells.J Virol. 2007 Feb;81(4):1610-8. doi: 10.1128/JVI.01433-06. Epub 2006 Nov 29. J Virol. 2007. PMID: 17135322 Free PMC article.
References
-
- Arias, C. F., P. Isa, C. A. Guerrero, E. Mendez, S. Zarate, T. Lopez, R. Espinosa, P. Romero, and S. Lopez. 2002. Molecular biology of rotavirus cell entry. Arch. Med. Res. 33:356-361. - PubMed
-
- Bass, D. M., M. Baylor, C. Chen, and U. Upadhyayula. 1995. Dansylcadaverine and cytochalasin D enhance rotavirus infection of murine L cells. Virology 212:429-437. - PubMed
-
- Bowden, D. S., J. S. Pedersen, B. H. Toh, and E. G. Westaway. 1987. Distribution by immunofluorescence of viral products and actin-containing cytoskeletal filaments in rubella virus-infected cells. Arch. Virol. 92:211-219. - PubMed
-
- Bretscher, A. 1999. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 11:109-116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

