Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies
- PMID: 16571816
- PMCID: PMC1440468
- DOI: 10.1128/JVI.80.8.3994-4004.2006
Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies
Abstract
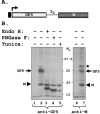
Porcine reproductive and respiratory syndrome virus (PRRSV) glycoprotein 5 (GP5) is the most abundant envelope glycoprotein and a major inducer of neutralizing antibodies in vivo. Three putative N-linked glycosylation sites (N34, N44, and N51) are located on the GP5 ectodomain, where a major neutralization epitope also exists. To determine which of these putative sites are used for glycosylation and the role of the glycan moieties in the neutralizing antibody response, we generated a panel of GP5 mutants containing amino acid substitutions at these sites. Biochemical studies with expressed wild-type (wt) and mutant proteins revealed that the mature GP5 contains high-mannose-type sugar moieties at all three sites. These mutations were subsequently incorporated into a full-length cDNA clone. Our data demonstrate that mutations involving residue N44 did not result in infectious progeny production, indicating that N44 is the most critical amino acid residue for infectivity. Viruses carrying mutations at N34, N51, and N34/51 grew to lower titers than the wt PRRSV. In serum neutralization assays, the mutant viruses exhibited enhanced sensitivity to neutralization by wt PRRSV-specific antibodies. Furthermore, inoculation of pigs with the mutant viruses induced significantly higher levels of neutralizing antibodies against the mutant as well as the wt PRRSV, suggesting that the loss of glycan residues in the ectodomain of GP5 enhances both the sensitivity of these viruses to in vitro neutralization and the immunogenicity of the nearby neutralization epitope. These results should have great significance for development of PRRSV vaccines of enhanced protective efficacy.
Figures


References
-
- Alexander, S., and J. H. Elder. 1984. Carbohydrate dramatically influences immune reactivity of antisera to viral glycoprotein antigens. Science 226:1328-1330. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. D. Seidman, J. A. Smith, and K. Struhl (ed.). 2001. Current protocols in molecular biology, 2nd ed. John Wiley and Sons, New York, N.Y.
-
- Bolmstedt, A., S. Sjolander, J. E. Hansen, L. Akerblom, A. Hemming, S. L. Hu, B. Morein, and S. Olofsson. 1996. Influence of N-linked glycans in V4-V5 region of human immunodeficiency virus type 1 glycoprotein gp160 on induction of a virus-neutralizing humoral response. J. Acquir. Immune Defic. Syndr. Hum. Retrovir. 12:213-220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

