Functional analysis of the tick-borne encephalitis virus cyclization elements indicates major differences between mosquito-borne and tick-borne flaviviruses
- PMID: 16571826
- PMCID: PMC1440478
- DOI: 10.1128/JVI.80.8.4099-4113.2006
Functional analysis of the tick-borne encephalitis virus cyclization elements indicates major differences between mosquito-borne and tick-borne flaviviruses
Abstract
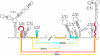
The linear, positive-stranded RNA genome of flaviviruses is thought to adopt a circularized conformation via interactions of short complementary sequence elements located within its terminal regions. This process of RNA cyclization is a crucial precondition for RNA replication. In the case of mosquito-borne flaviviruses, highly conserved cyclization sequences (CS) have been identified, and their functionality has been experimentally confirmed. Here, we provide an experimental identification of CS elements of tick-borne encephalitis virus (TBEV). These elements, termed 5'-CS-A and 3'-CS-A, are conserved among various tick-borne flaviviruses, but they are unrelated to the mosquito-borne CS elements and are located at different genomic positions. The 5'-CS-A element is situated upstream rather than downstream of the AUG start codon and, in contrast to mosquito-borne flaviviruses, it was found that the entire protein C coding region is not essential for TBEV replication. The complementary 3'-CS-A element is located within the bottom stem rather than upstream of the characteristic 3'-terminal stem-loop structure, implying that this part of the proposed structure cannot be formed when the genome is in its circularized conformation. Finally, we demonstrate that the CS-A elements can also mediate their function when the 5'-CS-A element is moved from its natural position to one corresponding to the mosquito-borne CS. The recognition of essential RNA elements and their differences between mosquito-borne and tick-borne flaviviruses has practical implications for the design of replicons in vaccine and vector development.
Figures




Similar articles
-
Complete genomic sequence of Powassan virus: evaluation of genetic elements in tick-borne versus mosquito-borne flaviviruses.Virology. 1993 May;194(1):173-84. doi: 10.1006/viro.1993.1247. Virology. 1993. PMID: 8097605
-
Rapid identification of vector-borne flaviviruses by mass spectrometry.Mol Cell Probes. 2010 Aug;24(4):219-28. doi: 10.1016/j.mcp.2010.04.003. Epub 2010 Apr 20. Mol Cell Probes. 2010. PMID: 20412852
-
Essential role of cyclization sequences in flavivirus RNA replication.J Virol. 2001 Jul;75(14):6719-28. doi: 10.1128/JVI.75.14.6719-6728.2001. J Virol. 2001. PMID: 11413342 Free PMC article.
-
Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis.Virus Res. 2005 Aug;111(2):161-74. doi: 10.1016/j.virusres.2005.04.007. Virus Res. 2005. PMID: 15871909 Review.
-
Functional non-coding RNAs derived from the flavivirus 3' untranslated region.Virus Res. 2015 Aug 3;206:53-61. doi: 10.1016/j.virusres.2015.01.026. Epub 2015 Feb 7. Virus Res. 2015. PMID: 25660582 Review.
Cited by
-
Structures and Functions of the 3' Untranslated Regions of Positive-Sense Single-Stranded RNA Viruses Infecting Humans and Animals.Front Cell Infect Microbiol. 2020 Aug 27;10:453. doi: 10.3389/fcimb.2020.00453. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32974223 Free PMC article. Review.
-
Ixodes ricinus as potential vector for Usutu virus.PLoS Negl Trop Dis. 2024 Jul 10;18(7):e0012172. doi: 10.1371/journal.pntd.0012172. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38985837 Free PMC article.
-
A 5' RNA element promotes dengue virus RNA synthesis on a circular genome.Genes Dev. 2006 Aug 15;20(16):2238-49. doi: 10.1101/gad.1444206. Epub 2006 Aug 1. Genes Dev. 2006. PMID: 16882970 Free PMC article.
-
The role of viral persistence in flavivirus biology.Pathog Dis. 2014 Jul;71(2):137-63. doi: 10.1111/2049-632X.12178. Epub 2014 May 12. Pathog Dis. 2014. PMID: 24737600 Free PMC article. Review.
-
Conserved long-range interactions are required for stable folding of orthoflaviviral genomic RNA.Nucleic Acids Res. 2025 Jun 6;53(11):gkaf514. doi: 10.1093/nar/gkaf514. Nucleic Acids Res. 2025. PMID: 40521663 Free PMC article.
References
-
- Ackermann, M., and R. Padmanabhan. 2001. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276:39926-39937. - PubMed
-
- Alvarez, D. E., A. L. Lella Ezcurra, S. Fucito, and A. V. Gamarnik. 2005. Role of RNA structures present at the 3′UTR of dengue virus on translation, RNA synthesis, and viral replication. Virology 339:200-212. - PubMed
-
- Blumenthal, T., and G. G. Carmichael. 1979. RNA replication: function and structure of Qbeta-replicase. Annu. Rev. Biochem. 48:525-548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

