Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein
- PMID: 16571833
- PMCID: PMC1440424
- DOI: 10.1128/JVI.80.8.4174-4178.2006
Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein
Abstract
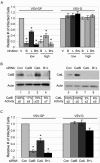
Using chemical inhibitors and small interfering RNA (siRNA), we have confirmed roles for cathepsin B (CatB) and cathepsin L (CatL) in Ebola virus glycoprotein (GP)-mediated infection. Treatment of Ebola virus GP pseudovirions with CatB and CatL converts GP1 from a 130-kDa to a 19-kDa species. Virus with 19-kDa GP1 displays significantly enhanced infection and is largely resistant to the effects of the CatB inhibitor and siRNA, but it still requires a low-pH-dependent endosomal/lysosomal function. These and other results support a model in which CatB and CatL prime GP by generating a 19-kDa intermediate that can be acted upon by an as yet unidentified endosomal/lysosomal enzyme to trigger fusion.
Figures
Similar articles
-
Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection.Science. 2005 Jun 10;308(5728):1643-5. doi: 10.1126/science.1110656. Epub 2005 Apr 14. Science. 2005. PMID: 15831716 Free PMC article.
-
Inhibitors of signal peptide peptidase and subtilisin/kexin-isozyme 1 inhibit Ebola virus glycoprotein-driven cell entry by interfering with activity and cellular localization of endosomal cathepsins.PLoS One. 2019 Apr 11;14(4):e0214968. doi: 10.1371/journal.pone.0214968. eCollection 2019. PLoS One. 2019. PMID: 30973897 Free PMC article.
-
[Ebola and Marburg viruses: the humans strike back].Med Sci (Paris). 2006 Apr;22(4):405-10. doi: 10.1051/medsci/2006224405. Med Sci (Paris). 2006. PMID: 16597410 Review. French.
-
Proteolysis of the Ebola virus glycoproteins enhances virus binding and infectivity.J Virol. 2007 Dec;81(24):13378-84. doi: 10.1128/JVI.01170-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928356 Free PMC article.
-
Towards specific functions of lysosomal cysteine peptidases: phenotypes of mice deficient for cathepsin B or cathepsin L.Biol Chem. 2001 May;382(5):735-41. doi: 10.1515/BC.2001.089. Biol Chem. 2001. PMID: 11517926 Review.
Cited by
-
Structural basis for Marburg virus neutralization by a cross-reactive human antibody.Cell. 2015 Feb 26;160(5):904-912. doi: 10.1016/j.cell.2015.01.041. Cell. 2015. PMID: 25723165 Free PMC article.
-
Inhibition of Ebola Virus Infection: Identification of Niemann-Pick C1 as the Target by Optimization of a Chemical Probe.ACS Med Chem Lett. 2013 Feb 14;4(2):239-243. doi: 10.1021/ml300370k. Epub 2012 Dec 19. ACS Med Chem Lett. 2013. PMID: 23526644 Free PMC article.
-
Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs.PLoS Pathog. 2015 Jun 26;11(6):e1005016. doi: 10.1371/journal.ppat.1005016. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26115029 Free PMC article.
-
Filovirus tropism: cellular molecules for viral entry.Front Microbiol. 2012 Feb 6;3:34. doi: 10.3389/fmicb.2012.00034. eCollection 2012. Front Microbiol. 2012. PMID: 22363323 Free PMC article.
-
Interaction between TIM-1 and NPC1 Is Important for Cellular Entry of Ebola Virus.J Virol. 2015 Jun;89(12):6481-93. doi: 10.1128/JVI.03156-14. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855742 Free PMC article.
References
-
- Ebert, D. H., J. Deussing, C. Peters, and T. S. Dermody. 2002. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277:24609-24617. - PubMed
-
- Golden, J., and L. Schiff. 2005. Neutrophil elastase, an acid-independent serine protease, facilitates reovirus uncoating and infection in U937 promonocyte cells. Virol. J. 2:48. http://www.virologyj.com/content/2/1/48. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

