Development of callosal topography in visual cortex of normal and enucleated rats
- PMID: 16572463
- PMCID: PMC2577613
- DOI: 10.1002/cne.20938
Development of callosal topography in visual cortex of normal and enucleated rats
Abstract
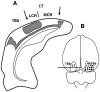
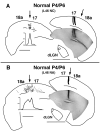
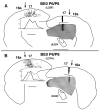
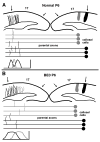
In normal rats callosal projections in striate cortex connect retinotopically corresponding, nonmirror-symmetric cortical loci, whereas in rats bilaterally enucleated at birth, callosal fibers connect topographically mismatched, mirror-symmetric loci. Moreover, retina input specifies the topography of callosal projections by postnatal day (P)6. To investigate whether retinal input guides development of callosal maps by promoting either the corrective pruning of exuberant axon branches or the specific ingrowth and elaboration of axon branches at topographically correct places, we studied the topography of emerging callosal connections at and immediately after P6. After restricted intracortical injections of anterogradely and retrogradely transported tracers we observed that the normal, nonmirror-symmetric callosal map, as well as the anomalous, mirror-symmetric map observed in neonatally enucleated animals, are present by P6-7, just as collateral branches of simple architecture emerge from their parental axons and grow into superficial cortical layers. Our results therefore do not support the idea that retinal input guides callosal map formation by primarily promoting the large-scale elimination of long, nontopographic branches and arbors. Instead, they suggest that retinal input specifies the sites on the parental axons from which interstitial branches will grow to invade middle and upper cortical layers, thereby ensuring that the location of invading interstitial branches is accurately related to the topographical location of the soma that gives rise to the parental axon. Moreover, our results from enucleated rats suggest that the cues that determine the mirror-symmetric callosal map exert only a weak control on the topography of fiber ingrowth.
Figures






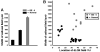

Similar articles
-
Retinal influences specify cortico-cortical maps by postnatal day six in rats and mice.J Comp Neurol. 2003 Apr 28;459(2):156-72. doi: 10.1002/cne.10615. J Comp Neurol. 2003. PMID: 12640667
-
Effects of neonatal enucleation on the organization of callosal linkages in striate cortex of the rat.J Comp Neurol. 1995 Oct 9;361(1):138-51. doi: 10.1002/cne.903610111. J Comp Neurol. 1995. PMID: 8550875
-
Role of retinal input on the development of striate-extrastriate patterns of connections in the rat.J Comp Neurol. 2012 Oct 1;520(14):3256-76. doi: 10.1002/cne.23096. J Comp Neurol. 2012. PMID: 22430936 Free PMC article.
-
Visual inter-hemispheric processing: constraints and potentialities set by axonal morphology.J Physiol Paris. 1999 Sep-Oct;93(4):271-84. doi: 10.1016/s0928-4257(00)80056-x. J Physiol Paris. 1999. PMID: 10574117 Review.
-
Cellular aspects of callosal connections and their development.Neuropsychologia. 1995 Aug;33(8):961-87. doi: 10.1016/0028-3932(95)00033-y. Neuropsychologia. 1995. PMID: 8524456 Review.
Cited by
-
Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity.J Neurosci. 2007 Jun 20;27(25):6760-70. doi: 10.1523/JNEUROSCI.1215-07.2007. J Neurosci. 2007. PMID: 17581963 Free PMC article.
-
Role of interstitial branching in the development of visual corticocortical connections: a time-lapse and fixed-tissue analysis.J Comp Neurol. 2010 Dec 15;518(24):4963-79. doi: 10.1002/cne.22502. J Comp Neurol. 2010. PMID: 21031561 Free PMC article.
-
Neonatal enucleation during a critical period reduces the precision of cortico-cortical projections in visual cortex.Neurosci Lett. 2011 Sep 1;501(3):152-6. doi: 10.1016/j.neulet.2011.07.005. Epub 2011 Jul 18. Neurosci Lett. 2011. PMID: 21782890 Free PMC article.
-
Deafferentation-induced plasticity of visual callosal connections: predicting critical periods and analyzing cortical abnormalities using diffusion tensor imaging.Neural Plast. 2012;2012:250196. doi: 10.1155/2012/250196. Epub 2012 Nov 8. Neural Plast. 2012. PMID: 23213572 Free PMC article. Review.
-
Activity-dependent callosal axon projections in neonatal mouse cerebral cortex.Neural Plast. 2012;2012:797295. doi: 10.1155/2012/797295. Epub 2012 Nov 19. Neural Plast. 2012. PMID: 23213574 Free PMC article. Review.
References
-
- Aggoun-Zouaoui D, Innocenti GM. Juvenile visual callosal axons in kittens display origin-and fate-related morphology and distribution of arbors. Eur J Neurosci. 1994;6:1846–1863. - PubMed
-
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. - PubMed
-
- Barone P, Dehay C, Berland M, Kennedy H. Role of directed growth and target selection in the formation of cortical pathways: prenatal development of the projection of area V2 to area V4 in the monkey. J Comp Neurol. 1996;374:1–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

