Molecular cloning of the m-Golsyn gene and its expression in the mouse brain
- PMID: 16572588
- PMCID: PMC6032447
- DOI: 10.3727/000000006783991917
Molecular cloning of the m-Golsyn gene and its expression in the mouse brain
Abstract
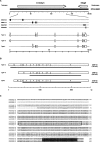
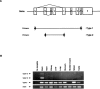
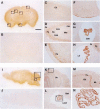
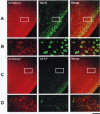
The mouse ortholog of the human GOLSYN gene, termed the m-Golsyn gene, was isolated and mapped to the region on mouse chromosome 15B3.2 syntenic with human chromosome 8q23. Three mRNA species (type la, 1b, and type 2) were produced by use of alternative transcription initiation points and alternative splicing events. The type 1 mRNAs were expressed only in the brain, whereas the type 2 was detected in various tissues. m-Golsyn protein was expressed in various tissues including the brain. Immunohistochemical study of m-Golsyn protein showed its prominent expression in the neuronal cells in various regions of the brain and strong expression in the choroid plexus ependymal cells lining the ventricles. m-Golsyn protein was found to be homologous to syntaphilin, a regulator of synaptic vesicle exocytosis. These results indicate that the m-Golsyn protein may play an important role in intracellular protein transport in neuronal cells of the brain.
Figures


Similar articles
-
Molecular cloning and characterization of gene for Golgi-localized syntaphilin-related protein on human chromosome 8q23.Gene. 2005 Jan 3;344:259-71. doi: 10.1016/j.gene.2004.10.024. Epub 2004 Dec 10. Gene. 2005. PMID: 15656992
-
Expression of m-Golsyn/Syntabulin gene during mouse brain development.Neurosci Lett. 2006 Aug 7;403(3):244-9. doi: 10.1016/j.neulet.2006.04.061. Epub 2006 Jun 5. Neurosci Lett. 2006. PMID: 16750881
-
Cloning, chromosome mapping and functional characterization of a human homologue of murine gtse-1 (B99) gene.Gene. 2000 Aug 22;254(1-2):229-36. doi: 10.1016/s0378-1119(00)00260-2. Gene. 2000. PMID: 10974554
-
Genomic organization of the mouse Msh4 gene producing bicistronic, chimeric and antisense mRNA.Gene. 2004 Nov 10;342(1):165-77. doi: 10.1016/j.gene.2004.08.016. Gene. 2004. PMID: 15527976
-
Cloning and characterization of Disc1, the mouse ortholog of DISC1 (Disrupted-in-Schizophrenia 1).Genomics. 2002 Dec;80(6):662-72. doi: 10.1006/geno.2002.7012. Genomics. 2002. PMID: 12504857
Cited by
-
Syntabulin regulates the trafficking of PICK1-containing vesicles in neurons.Sci Rep. 2016 Feb 12;6:20924. doi: 10.1038/srep20924. Sci Rep. 2016. PMID: 26868290 Free PMC article.
-
Uncovering molecular biomarkers that correlate cognitive decline with the changes of hippocampus' gene expression profiles in Alzheimer's disease.PLoS One. 2010 Apr 13;5(4):e10153. doi: 10.1371/journal.pone.0010153. PLoS One. 2010. PMID: 20405009 Free PMC article.
References
-
- Altschul S. F.; Gish W.; Miller W.; Myers E. W.; Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 215:403–410; 1990. - PubMed
-
- Chakrabortty S.; Kitada M.; Matsumoto N.; Taketomi M.; Kimura K.; Ide C. Choroid plexus ependymal cells enhance neurite outgrowth from dorsal root ganglion neurons in vitro. J. Neurocytol. 29:707–717; 2000. - PubMed
-
- Debus E.; Weber K.; Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation 25:193–203; 1983. - PubMed
-
- Dynan W. S.; Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. Nature 316:774–778; 1985. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
