Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4
- PMID: 16572725
- PMCID: PMC1400610
- DOI: 10.1379/csc-138r.1
Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4
Abstract
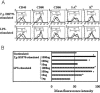
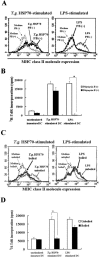
Toxoplasma gondii-derived heat shock protein 70 (T.g.HSP70) induced maturation of bone marrow-derived dendritic cells (DCs) of wild-type (WT) C57BL/6 mice as evidenced by an increase in surface expression of MHC class I and II molecules and costimulatory molecules such as CD40, CD80, and CD86. Functionally, decreased phagocytic ability and increased alloreactive T cell stimulatory ability were observed in T.g.HSP70-stimulated DCs. These phenotypic and functional changes of T.g.HSP70-stimulated DCs were demonstrated in Toll-like receptor (TLR) 2- and myeloid differentiation factor 88 (MyD88)-deficient but not TLR4-deficient C57BL/6 mice. DCs from WT and TLR2-deficient but not TLR4-deficient mice produced IL-12 after T.g.HSP70 stimulation. T.g.HSP70-stimulated DCs from WT, TLR2-deficient, and MyD88-deficient, but not TLR4-deficient mice expressed IFN-beta mRNA. Thus, T.g.HSP70 stimulates murine DC maturation via TLR4 through the MyD88-independent signal transduction cascade.
Figures

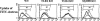
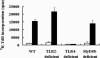

References
-
- Adachi O, Kawai T, and Takeda K. et al. 1998 Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 9:143–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
