Induction of the 72-kilodalton heat shock protein and protection from ultraviolet B-induced cell death in human keratinocytes by repetitive exposure to heat shock or 15-deoxy-delta(12,14)-prostaglandin J2
- PMID: 16572732
- PMCID: PMC1400615
- DOI: 10.1379/csc-89r.1
Induction of the 72-kilodalton heat shock protein and protection from ultraviolet B-induced cell death in human keratinocytes by repetitive exposure to heat shock or 15-deoxy-delta(12,14)-prostaglandin J2
Abstract
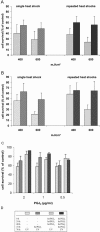
It has been demonstrated that hyperthermia protects keratinocytes from ultraviolet B (UVB)-induced cell death in culture and in vivo. This effect is mediated by the antiapoptotic effect of heat shock proteins that are transiently induced after exposure to heat at sublethal temperatures. Consequently, induction of Hsp has been proposed as a novel means of photoprotection. However, in the face of daily UVB exposure of human skin in vivo, this approach would not be useful if keratinocytes become less sensitive to Hsp induction with repeated exposure to the inducing agent. The aim of this study was to investigate whether repeated exposure to hyperthermia or to the stress protein activating cyclopentenone prostaglandin 15-deoxy-delta(12,14)-prostaglandin J2 (15dPGJ2) leads to adaptation of the cells, attenuation of the heat shock response, and abrogation of the protective effect. Normal human epidermal keratinocytes (NHEK) and the carcinoma-derived cell line A431 were exposed to either 42 degrees C or to 15dPGJ2 for 4 hours at 24-hour intervals for 4 consecutive days. The intracellular level of the 72-kDa heat shock protein (Hsp72) was determined by enzyme-linked immunosorbent assay (ELISA). Cells were exposed to UVB from a metal halide source after the last heat or 15dPGJ2 treatment, and survival was determined 24 hours after exposure by a MTT assay. Our results demonstrate that (1) heat shock and 15dPGJ2 are potent inducers of Hsp72 expression and lead to increased resistance to UVB-induced cell death in human keratinocytes; (2) re-exposure to heat shock leads to a superinduction without attenuation of the absolute increase in Hsp72 and of its UVB-protective effect; (3) the UVB tolerance induced by 15dPGJ2 is enhanced by repeated exposure without a further increase of Hsp72; (4) repeated heat shock and 15dPGJ2 up to a concentration of 1 microg/mL have no influence on cell growth over a period of 4 days. We conclude that through repeated exposure to Hsp-inducing factors, stress tolerance can be maintained without additional toxicity in human keratinocytes. These results provide a basis for the development of nontoxic Hsp inducers that can be repeatedly applied without loss of effect.
Figures


Similar articles
-
Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-delta12,14-prostaglandin J2 in human mesangial cells.Kidney Int. 2004 Mar;65(3):798-810. doi: 10.1111/j.1523-1755.2004.00454.x. Kidney Int. 2004. PMID: 14871400
-
72-kD heat shock protein is a mediator of resistance to ultraviolet B light.J Invest Dermatol. 1995 Aug;105(2):160-2. doi: 10.1111/1523-1747.ep12317003. J Invest Dermatol. 1995. PMID: 7636297
-
Heat shock transcription factor-1 regulates heat shock protein-72 expression in human keratinocytes exposed to ultraviolet B light.J Invest Dermatol. 1998 Aug;111(2):194-8. doi: 10.1046/j.1523-1747.1998.00266.x. J Invest Dermatol. 1998. PMID: 9699716
-
Significance of heat shock proteins in the skin upon UV exposure.Front Biosci (Landmark Ed). 2009 Jan 1;14(12):4758-68. doi: 10.2741/3565. Front Biosci (Landmark Ed). 2009. PMID: 19273387 Review.
-
Heat shock and UV-B-induced DNA damage and mutagenesis in skin.Photochem Photobiol Sci. 2003 Sep;2(9):899-903. doi: 10.1039/b301253k. Photochem Photobiol Sci. 2003. PMID: 14560806 Review.
Cited by
-
The heat shock protein response following eccentric exercise in human skeletal muscle is unaffected by local NSAID infusion.Eur J Appl Physiol. 2013 Jul;113(7):1883-93. doi: 10.1007/s00421-013-2606-y. Epub 2013 Mar 7. Eur J Appl Physiol. 2013. PMID: 23467900
-
The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells.Cell Stress Chaperones. 2010 May;15(3):309-22. doi: 10.1007/s12192-009-0145-6. Epub 2009 Oct 7. Cell Stress Chaperones. 2010. PMID: 19809895 Free PMC article.
-
Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines.J Leukoc Biol. 2007 May;81(5):1179-87. doi: 10.1189/jlb.0506347. Epub 2007 Feb 20. J Leukoc Biol. 2007. PMID: 17311933 Free PMC article.
-
Heat shock proteins in the physiology and pathophysiology of epidermal keratinocytes.Cell Stress Chaperones. 2019 Nov;24(6):1027-1044. doi: 10.1007/s12192-019-01044-5. Epub 2019 Nov 16. Cell Stress Chaperones. 2019. PMID: 31734893 Free PMC article. Review.
-
The HSP90 inhibitor NVP-AUY922 radiosensitizes by abrogation of homologous recombination resulting in mitotic entry with unresolved DNA damage.PLoS One. 2012;7(4):e35436. doi: 10.1371/journal.pone.0035436. Epub 2012 Apr 16. PLoS One. 2012. PMID: 22523597 Free PMC article.
References
-
- Hamel L, Kenney M, Jayyosi Z, Ardati A, Clark K, Spada A, Zilberstein A, Perrone M, Kaplow J, Merkel L, Rojas C. Induction of heat shock protein 70 by herbimycin A and cyclopentenone prostaglandins in smooth muscle cells. Cell Stress Chaperones. 2000;5:121–131.1466-1268(2000)005[0121:IOHSPB]2.0.CO;2 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
