Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25
- PMID: 16573824
- PMCID: PMC1444931
- DOI: 10.1186/1741-7007-4-7
Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25
Abstract
Background: Taste receptor cells are responsible for transducing chemical stimuli from the environment and relaying information to the nervous system. Bitter, sweet and umami stimuli utilize G-protein coupled receptors which activate the phospholipase C (PLC) signaling pathway in Type II taste cells. However, it is not known how these cells communicate with the nervous system. Previous studies have shown that the subset of taste cells that expresses the T2R bitter receptors lack voltage-gated Ca2+ channels, which are normally required for synaptic transmission at conventional synapses. Here we use two lines of transgenic mice expressing green fluorescent protein (GFP) from two taste-specific promoters to examine Ca2+ signaling in subsets of Type II cells: T1R3-GFP mice were used to identify sweet- and umami-sensitive taste cells, while TRPM5-GFP mice were used to identify all cells that utilize the PLC signaling pathway for transduction. Voltage-gated Ca2+ currents were assessed with Ca2+ imaging and whole cell recording, while immunocytochemistry was used to detect expression of SNAP-25, a presynaptic SNARE protein that is associated with conventional synapses in taste cells.
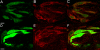
Results: Depolarization with high K+ resulted in an increase in intracellular Ca2+ in a small subset of non-GFP labeled cells of both transgenic mouse lines. In contrast, no depolarization-evoked Ca2+ responses were observed in GFP-expressing taste cells of either genotype, but GFP-labeled cells responded to the PLC activator m-3M3FBS, suggesting that these cells were viable. Whole cell recording indicated that the GFP-labeled cells of both genotypes had small voltage-dependent Na+ and K+ currents, but no evidence of Ca2+ currents. A subset of non-GFP labeled taste cells exhibited large voltage-dependent Na+ and K+ currents and a high threshold voltage-gated Ca2+ current. Immunocytochemistry indicated that SNAP-25 was expressed in a separate population of taste cells from those expressing T1R3 or TRPM5. These data indicate that G protein-coupled taste receptors and conventional synaptic signaling mechanisms are expressed in separate populations of taste cells.
Conclusion: The taste receptor cells responsible for the transduction of bitter, sweet, and umami stimuli are unlikely to communicate with nerve fibers by using conventional chemical synapses.
Figures
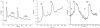



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

