Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases
- PMID: 16573826
- PMCID: PMC1562425
- DOI: 10.1186/1471-2334-6-66
Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases
Abstract
Background: New tools are required to improve tuberculosis (TB) diagnosis and treatment, including enhanced ability to compare new treatment strategies. The ELISPOT assay uses Mycobacterium tuberculosis-specific antigens to produce a precise quantitative readout of the immune response to pathogen. We hypothesized that TB patients in The Gambia would have reduced ELISPOT counts after successful treatment.
Methods: We recruited Gambian adults with sputum smear and culture positive tuberculosis for ELISPOT assay and HIV test, and followed them up one year later to repeat testing and document treatment outcome. We used ESAT-6, CFP-10 and Purified Protein Derivative (PPD) as stimulatory antigens. We confirmed the reliability of our assay in 23 volunteers through 2 tests one week apart, comparing within and between subject variation.
Results: We performed an ELISPOT test at diagnosis and 12 months later in 89 patients. At recruitment, 70/85 HIV-negative patients (82%) were ESAT-6 or CFP-10 (EC) ELISPOT positive, 77 (90%) were PPD ELISPOT positive. Eighty-two cases (96%) successfully completed treatment: 44 (55%; p < 0.001) were EC ELISPOT negative at 12 months, 17 (21%; p = 0.051) were PPD ELISPOT negative. Sixty (73%) cured cases had a CFP-10 ELISPOT count decrease, 64 (78%) had an ESAT-6 ELISPOT count decrease, 58 (70%) had a PPD ELISPOT count decrease. There was a mean decline of 25, 44 and 47 SFU/2 x 105 cells for CFP-10, ESAT-6 and PPD respectively (p < 0.001 for all). Three of 4 HIV positive patients were cured, all 3 underwent ELISPOT reversion; all 4 not cured subjects (3 HIV-negative, 1 HIV positive) were ESAT-6, CFP-10 and PPD ELISPOT positive at 12 months.
Conclusion: Successful tuberculosis treatment is accompanied by a significant reduction in the M. tuberculosis-specific antigen ELISPOT count. The ELISPOT has potential as a proxy measure of TB treatment outcome. Further investigation into the decay kinetics of T-cells with treatment is warranted.
Figures
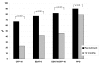

Similar articles
-
Reversion and conversion of Mycobacterium tuberculosis IFN-gamma ELISpot results during anti-tuberculous treatment in HIV-infected children.BMC Infect Dis. 2010 May 27;10:138. doi: 10.1186/1471-2334-10-138. BMC Infect Dis. 2010. PMID: 20507549 Free PMC article.
-
Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model.Clin Infect Dis. 2005 Jan 15;40(2):273-8. doi: 10.1086/427030. Epub 2004 Dec 20. Clin Infect Dis. 2005. PMID: 15655747
-
Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia.Clin Infect Dis. 2004 Apr 1;38(7):966-73. doi: 10.1086/382362. Epub 2004 Mar 16. Clin Infect Dis. 2004. PMID: 15034828
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
[Characteristics of a diagnostic method for tuberculosis infection based on whole blood interferon-gamma assay].Kekkaku. 2006 Nov;81(11):681-6. Kekkaku. 2006. PMID: 17154047 Review. Japanese.
Cited by
-
In vivo and in vitro effects of antituberculosis treatment on mycobacterial interferon-gamma T cell response.PLoS One. 2009;4(4):e5187. doi: 10.1371/journal.pone.0005187. Epub 2009 Apr 13. PLoS One. 2009. PMID: 19365543 Free PMC article.
-
New Insights into Biomarkers for Evaluating Therapy Efficacy in Pulmonary Tuberculosis: A Narrative Review.Infect Dis Ther. 2023 Dec;12(12):2665-2689. doi: 10.1007/s40121-023-00887-x. Epub 2023 Nov 8. Infect Dis Ther. 2023. PMID: 37938418 Free PMC article. Review.
-
IP-10 response to RD1 antigens might be a useful biomarker for monitoring tuberculosis therapy.BMC Infect Dis. 2011 May 19;11:135. doi: 10.1186/1471-2334-11-135. BMC Infect Dis. 2011. PMID: 21595874 Free PMC article.
-
Kinetics of T-cell-based assays on cerebrospinal fluid and peripheral blood mononuclear cells in patients with tuberculous meningitis.Korean J Intern Med. 2014 Nov;29(6):793-9. doi: 10.3904/kjim.2014.29.6.793. Epub 2014 Oct 31. Korean J Intern Med. 2014. PMID: 25378978 Free PMC article.
-
Reversion and conversion of Mycobacterium tuberculosis IFN-gamma ELISpot results during anti-tuberculous treatment in HIV-infected children.BMC Infect Dis. 2010 May 27;10:138. doi: 10.1186/1471-2334-10-138. BMC Infect Dis. 2010. PMID: 20507549 Free PMC article.
References
-
- WHO report. Geneva, World Health Organisation (WHO/HTM/TB/2005.349); 2005. Global tuberculosis control: sureveillance, planning, financing.
-
- Hill PC, Fox A, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, Donkor SA, Hammond AS, Corrah T, Adegbola RA, McAdam KPWJ, Brookes RH. Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin Infect Dis. 2005;40:273–8. doi: 10.1086/427030. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

