Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots
- PMID: 16574693
- PMCID: PMC2803390
- DOI: 10.1093/aob/mcl063
Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots
Abstract
Aims: Arbuscular mycorrhizae are formed between >80 % of land plants and arbuscular mycorrhizal (AM) fungi. This Botanical Briefing highlights the chemical identification of strigolactones as a host-recognition signal for AM fungi, and their role in the establishment of arbuscular mycorrhizae as well as in the seed germination of parasitic weeds.
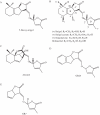
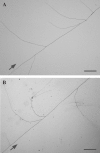
Scope: Hyphal branching has long been described as the first morphological event in host recognition by AM fungi during the pre-infection stages. Host roots release signalling molecules called 'branching factors' that induce extensive hyphal branching in AM fungi. Strigolactones exuded from host roots have recently been identified as an inducer of hyphal branching in AM fungi. Strigolactones are a group of sesquiterpenes, previously isolated as seed germination stimulants for the parasitic weeds Striga and Orobanche. Parasitic weeds might find their potential hosts by detecting strigolactones, which are released from plant roots upon phosphate deficiency in communication with AM fungi. In addition to acting as a signalling molecule, strigolactones might stimulate the production of fungal symbiotic signals called 'Myc factors' in AM fungi.
Conclusions: Isolation and identification of plant symbiotic signals open up new ways for studying the molecular basis of plant-AM-fungus interactions. This discovery provides a clear answer to a long-standing question in parasitic plant biology: what is the natural role for germination stimulants? It could also provide a new strategy for the management and control of beneficial fungal symbionts and of devastating parasitic weeds in agriculture and natural ecosystems.
Figures

References
-
- Akiyama K, Matsuzaki K. Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827. - PubMed
-
- Bécard G, Piché Y. 1989. New aspects on the acquisition of biotrophic status by a vesicular-arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytologist 112: 77–83.
-
- Bécard G, Taylor LP, Douds DD, Pfeffer PE, Doner LW. 1995. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. Molecular Plant-Microbe Interactions 8: 252–258.
-
- Bécard G, Roux C, Sejalon-Delmas N, Puech V, Sébastien R. 2005. Modulators of the development of mycorrhizal fungi with arbuscules, and uses thereof. WO Patent 2005/077177 A2.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

