Turing instability mediated by voltage and calcium diffusion in paced cardiac cells
- PMID: 16574775
- PMCID: PMC1458631
- DOI: 10.1073/pnas.0511061103
Turing instability mediated by voltage and calcium diffusion in paced cardiac cells
Abstract
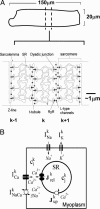
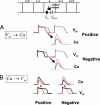
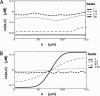
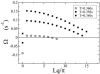
In cardiac cells, the coupling between the voltage across the cell membrane (V(m)) and the release of calcium (Ca) from intracellular stores is a crucial ingredient of heart function. Under abnormal conditions and/or rapid pacing, both the action potential duration and the peak Ca concentration in the cell can exhibit well known period-doubling oscillations referred to as "alternans," which have been linked to sudden cardiac death. Fast diffusion of V(m) keeps action potential duration alternans spatially synchronized over the approximately 150-mum-length scale of a cell, but slow diffusion of Ca ions allows Ca alternans within a cell to become spatially asynchronous, as observed in some experiments. This finding raises the question: When are Ca alternans spatially in-phase or out-of-phase on subcellular length scales? This question is investigated by using a spatially distributed model of Ca cycling coupled to V(m). Our main finding is the existence of a Turing-type symmetry breaking instability mediated by V(m) and Ca diffusion that causes Ca alternans to become spontaneously out-of-phase at opposite ends of a cardiac cell. Pattern formation is governed by the interplay of short-range activation of Ca alternans, because of a dynamical instability of Ca cycling, and long-range inhibition of Ca alternans by V(m) alternans through Ca-sensitive membrane ionic currents. These results provide a striking example of a Turing instability in a biological context where the morphogens can be clearly identified, as well as a potential link between dynamical instability on subcellular scales and life-threatening cardiac disorders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

