Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease
- PMID: 16574934
- PMCID: PMC2662920
- DOI: 10.1164/rccm.200509-1461OC
Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease
Abstract
Rationale: Interactions of nontypeable Haemophilus influenzae (NTHI) with macrophages are implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). However, the immunologic mechanisms that mediate NTHI-macrophage inflammation are poorly understood. Outer membrane protein (OMP) P6 and lipooligosaccharide (LOS) of NTHI are potent immunomodulators. We theorized that alveolar macrophages in COPD possess fundamental immune defects that permit NTHI to evade host responses.
Objective: To test this hypothesis, we obtained human alveolar and blood macrophages from exsmokers with COPD, exsmokers without COPD, and nonsmokers.
Methods: Alveolar and blood macrophages from each donor were incubated with purified LOS and OMP P6 and with OMP P2 and the total outer membrane preparation (0.1-1 microg/ml).
Measurements: Supernatants (24 h) were assayed for IL-1beta, TNF-alpha, IL-10, IL-12, and IL-8 by multianalyte multiplexed flow cytometry.
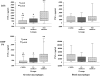
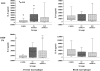
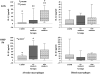
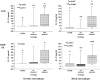
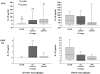
Results: Comparative induction of COPD and non-COPD alveolar macrophages by LOS and OMP P6 revealed diminished IL-8, TNF-alpha, and IL-1beta responses of COPD alveolar macrophages (p < or = 0.03 for each). COPD alveolar macrophages also had diminished responses to total outer membrane (p < or = 0.03 for each). In contrast, COPD blood macrophages had no significant differences among donor groups in IL-8, TNF-alpha, or IL-1beta responsiveness to NTHI antigens. Diminished IL-12 responses of COPD blood macrophages to NTHI antigens, compared with nonsmokers, could not be independently dissociated from group differences in age and pack-years.
Conclusions: These findings support a paradigm of defective immune responsiveness of alveolar macrophages, but not blood macrophages, in COPD.
Figures
Similar articles
-
Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines.Infect Immun. 2005 May;73(5):2728-35. doi: 10.1128/IAI.73.5.2728-2735.2005. Infect Immun. 2005. PMID: 15845475 Free PMC article.
-
Impaired phagocytosis of nontypeable Haemophilus influenzae by human alveolar macrophages in chronic obstructive pulmonary disease.J Infect Dis. 2006 Nov 15;194(10):1375-84. doi: 10.1086/508428. Epub 2006 Oct 16. J Infect Dis. 2006. PMID: 17054066
-
Calcium restores the macrophage response to nontypeable haemophilus influenzae in chronic obstructive pulmonary disease.Am J Respir Cell Mol Biol. 2015 Jun;52(6):728-37. doi: 10.1165/rcmb.2014-0172OC. Am J Respir Cell Mol Biol. 2015. PMID: 25338285
-
The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae.Front Immunol. 2018 Nov 5;9:2530. doi: 10.3389/fimmu.2018.02530. eCollection 2018. Front Immunol. 2018. PMID: 30455693 Free PMC article. Review.
-
Understanding nontypeable Haemophilus influenzae and chronic obstructive pulmonary disease.Curr Opin Pulm Med. 2014 Mar;20(2):159-64. doi: 10.1097/MCP.0000000000000023. Curr Opin Pulm Med. 2014. PMID: 24441573 Review.
Cited by
-
Lung macrophages drive mucus production and steroid-resistant inflammation in chronic bronchitis.Respir Res. 2021 Jun 7;22(1):172. doi: 10.1186/s12931-021-01762-4. Respir Res. 2021. PMID: 34098956 Free PMC article.
-
Immune response to pertussis vaccine in COPD patients.Sci Rep. 2023 Jul 19;13(1):11654. doi: 10.1038/s41598-023-38355-8. Sci Rep. 2023. PMID: 37468500 Free PMC article.
-
Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model.Sci Transl Med. 2011 Apr 13;3(78):78ra32. doi: 10.1126/scitranslmed.3002042. Sci Transl Med. 2011. PMID: 21490276 Free PMC article.
-
Large-scale, ion-current-based proteomics investigation of bronchoalveolar lavage fluid in chronic obstructive pulmonary disease patients.J Proteome Res. 2014 Feb 7;13(2):627-639. doi: 10.1021/pr4007602. Epub 2013 Dec 2. J Proteome Res. 2014. PMID: 24188068 Free PMC article.
-
Infections in chronic lung diseases.Infect Dis Clin North Am. 2007 Sep;21(3):673-95, viii. doi: 10.1016/j.idc.2007.06.006. Infect Dis Clin North Am. 2007. PMID: 17826618 Free PMC article. Review.
References
-
- Murphy TF, Apicella MA. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis 1987;9:1–15. - PubMed
-
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–471. - PubMed
-
- Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection: comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:947–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

