Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice
- PMID: 16574944
- PMCID: PMC1820635
- DOI: 10.1165/rcmb.2005-0471OC
Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice
Abstract
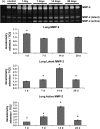
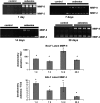
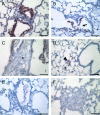
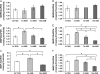
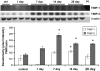
Inhalation of asbestos fibers causes pulmonary inflammation and eventual pulmonary fibrosis (asbestosis). Although the underlying molecular events are poorly understood, protease/antiprotease and oxidant/antioxidant imbalances are believed to contribute to the disease. Implicated in other forms of pulmonary fibrosis, the matrix metalloproteinases (MMPs) have not been examined in asbestosis. We therefore hypothesized that MMPs play a pathogenic role in asbestosis development. Wild-type C57BL/6 mice were intratracheally instilled with 0.1 mg crocidolite asbestos, causing an inflammatory response at 1 d and a developing fibrotic response at 7, 14, and 28 d. Gelatin zymography demonstrated an increase in MMP-9 (gelatinase B) during the inflammatory phase, while MMP-2 (gelatinase A) was profoundly increased in the fibrotic phase. Immunohistochemistry revealed MMP-9 in and around bronchiolar and airspace neutrophils that were often associated with visible asbestos fibers. MMP-2 was found in fibrotic regions at 7, 14, and 28 d. No increases in RNA levels of MMP-2, MMP-9, or MMP-8 were found, but levels of MMP-7, MMP-12, and MMP-13 RNA did increase at 14 d. The MMP inhibitors, TIMP-1 and TIMP-2, were also increased at 7-28 d after asbestos exposure. To confirm the importance of MMP activity in disease progression, mice exposed to asbestos were given daily injections of the MMP inhibitor, GM6001. MMP inhibition reduced inflammation and fibrosis in asbestos-treated mice. Collectively, these data suggest that MMPs contribute to the pathogenesis of asbestosis through effects on inflammation and fibrosis development.
Figures

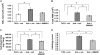
Similar articles
-
Redistribution of pulmonary EC-SOD after exposure to asbestos.J Appl Physiol (1985). 2004 Nov;97(5):2006-13. doi: 10.1152/japplphysiol.00480.2004. Epub 2004 Aug 6. J Appl Physiol (1985). 2004. PMID: 15298984
-
Alveolar macrophage cytokine and growth factor production in a rat model of crocidolite-induced pulmonary inflammation and fibrosis.J Toxicol Environ Health. 1995 Oct;46(2):155-69. doi: 10.1080/15287399509532026. J Toxicol Environ Health. 1995. PMID: 7563215
-
Unbalanced MMP/TIMP-1 expression during the development of experimental pulmonary fibrosis with acute paraquat poisoning.Mol Med Rep. 2011 Mar-Apr;4(2):243-8. doi: 10.3892/mmr.2011.425. Epub 2011 Jan 14. Mol Med Rep. 2011. PMID: 21468558
-
Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases.Eur J Pharmacol. 2006 Mar 8;533(1-3):133-44. doi: 10.1016/j.ejphar.2005.12.082. Epub 2006 Feb 20. Eur J Pharmacol. 2006. PMID: 16487964 Review.
-
Matrix metalloproteinase-9 in lung remodeling.Am J Respir Cell Mol Biol. 2003 Jan;28(1):12-24. doi: 10.1165/rcmb.2002-0166TR. Am J Respir Cell Mol Biol. 2003. PMID: 12495928 Review.
Cited by
-
Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients.Clin Cancer Res. 2012 May 1;18(9):2478-89. doi: 10.1158/1078-0432.CCR-11-2614. Epub 2012 Feb 27. Clin Cancer Res. 2012. PMID: 22371455 Free PMC article.
-
SP-1 regulation of MMP-9 expression requires Ser586 in the PEST domain.Biochem J. 2012 Jul 15;445(2):229-36. doi: 10.1042/BJ20120053. Biochem J. 2012. PMID: 22519702 Free PMC article.
-
The Versatile Role of Matrix Metalloproteinase for the Diverse Results of Fibrosis Treatment.Molecules. 2019 Nov 19;24(22):4188. doi: 10.3390/molecules24224188. Molecules. 2019. PMID: 31752262 Free PMC article. Review.
-
Inhibition of matrix metalloproteinase-9 attenuates kidney fibrosis and cellular senescence in the transition from acute kidney injury to chronic kidney disease.Ren Fail. 2025 Dec;47(1):2499897. doi: 10.1080/0886022X.2025.2499897. Epub 2025 Jun 4. Ren Fail. 2025. PMID: 40468751 Free PMC article.
-
Global gene profiling of aging lungs in Atp8b1 mutant mice.Aging (Albany NY). 2016 Sep 29;8(9):2232-2252. doi: 10.18632/aging.101056. Aging (Albany NY). 2016. PMID: 27689529 Free PMC article.
References
-
- Darcey DJ, Alleman T. Occupational and environmental exposure to asbestos. In: Roggli VL, Oury TD, Sporn TA, editors. Pathology of asbestos-associated diseases, 2nd ed. New York: Springer; 2004. pp. 17–33.
-
- Kamp DW, Weitzman SA. Asbestosis: clinical spectrum and pathogenic mechanisms. Proc Soc Exp Biol Med 1997;214:12–26. - PubMed
-
- Dai J, Churg A. Relationship of fiber surface iron and active oxygen species to expression of procollagen, PDGF-A, and TGF-beta(1) in tracheal explants exposed to amosite asbestos. Am J Respir Cell Mol Biol 2001;24:427–435. - PubMed
-
- Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol 2004;97:2006–2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

