Enhanced mixture analysis of poly(ethylene glycol) using high-field asymmetric waveform ion mobility spectrometry combined with fourier transform ion cyclotron resonance mass spectrometry
- PMID: 16579597
- PMCID: PMC2562220
- DOI: 10.1021/ac051709x
Enhanced mixture analysis of poly(ethylene glycol) using high-field asymmetric waveform ion mobility spectrometry combined with fourier transform ion cyclotron resonance mass spectrometry
Abstract
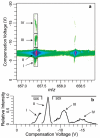
The combination of high-field asymmetric waveform ion mobility spectrometry (FAIMS) with Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) makes possible lower detection limits, increased sensitivity, and increased dynamic range in the analysis of poly(ethylene glycol) (PEG) samples of low molecular weight. The signal gain obtained using FAIMS depends on ion identity, with a range between 1.8x and 14x obtained for various molecular ions of PEG 600. A 1.7-fold reduction in noise is obtained using FAIMS due to the elimination of chemical noise. The improved detection performance is predominantly due to a reduction in adverse Coulomb effects as a result of ions being selectively introduced into the mass spectrometer. The high ion transmission obtained using FAIMS combined with the high sensitivity of FTICR-MS detection make possible separation of multiple gas-phase conformers of PEG molecular cations that have low abundance (less than 0.2% relative abundance) and that have not been detected previously. Mixed dications of PEG that have the same nominal mass but differ by the number polymer subunits (m/Delta m up to 25,000) can be separately introduced into the mass spectrometer using FAIMS. Interactions of the carrier gas with the metal ions that are attached to the PEG molecules appear to be the most significant factor in these FAIMS separations.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

