Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells
- PMID: 16579687
- PMCID: PMC4035024
- DOI: 10.1089/ten.2006.12.537
Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells
Abstract
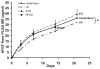
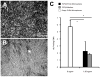
Despite the widespread role of transforming growth factor-beta3 (TGFbeta3) in wound healing and tissue regeneration, its long-term controlled release has not been demonstrated. Here, we report microencapsulation of TGFbeta3 in poly-d-l-lactic-co-glycolic acid (PLGA) microspheres and determine its bioactivity. The release profiles of PLGA-encapsulated TGFbeta3 with 50:50 and 75:25 PLA:PGA ratios differed throughout the experimental period. To compare sterilization modalities of microspheres, bFGF was encapsulated in 50:50 PLGA microspheres and subjected to ethylene oxide (EO) gas, radio-frequency glow discharge (RFGD), or ultraviolet (UV) light. The release of bFGF was significantly attenuated by UV light, but not significantly altered by either EO or RFGD. To verify its bioactivity, TGFbeta3 (1.35 ng/mL) was control-released to the culture of human mesenchymal stem cells (hMSC) under induced osteogenic differentiation. Alkaline phosphatase staining intensity was markedly reduced 1 week after exposing hMSC-derived osteogenic cells to TGFbeta3. This was confirmed by lower alkaline phosphatase activity (2.25 +/- 0.57 mU/mL/ng DNA) than controls (TGFbeta3- free) at 5.8 +/- 0.9 mU/mL/ng DNA (p < 0.05). Control-released TGFbeta3 bioactivity was further confirmed by lack of significant differences in alkaline phosphatase upon direct addition of 1.35 ng/mL TGFbeta3 to cell culture (p > 0.05). These findings provide baseline data for potential uses of microencapsulated TGFbeta3 in wound healing and tissue-engineering applications.
Figures



References
-
- Hosokawa R, Nonaka K, Morifuji M, Shum L, Ohishi M. TGF-beta 3 decreases type I collagen and scarring after labioplasty. J Dent Res. 2003;82:558. - PubMed
-
- Kohama K, Nonaka K, Hosokawa R, Shum L, Ohishi M. TGF-beta-3 promotes scarless repair of cleft lip in mouse fetuses. J Dent Res. 2002;81:688. - PubMed
-
- Cohen MM., Jr Craniofacial disorders caused by mutations in homeobox genes MSX1 and MSX2. J Craniofac Genet Dev Biol. 2000;20:19. - PubMed
-
- Opperman LA, Galanis V, Williams AR, Adab K. Transforming growth factor-beta3 (Tgf-beta3) down-regulates Tgf-beta3 receptor type I (Tbetar-I) during rescue of cranial sutures from osseous obliteration. Orthod Craniofac Res. 2002;5:5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

