Multiple functions of the integrin alpha6beta4 in epidermal homeostasis and tumorigenesis
- PMID: 16581764
- PMCID: PMC1446957
- DOI: 10.1128/MCB.26.8.2877-2886.2006
Multiple functions of the integrin alpha6beta4 in epidermal homeostasis and tumorigenesis
Figures
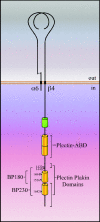

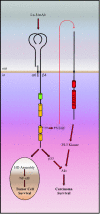
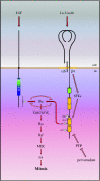
References
-
- Bertotti, A., P. M. Comoglio, and L. Trusolino. 2005. β4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res. 65:10674-10679. - PubMed
-
- Carter, W. G., M. C. Ryan, and P. J. Gahr. 1991. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell 65:599-610. - PubMed
-
- Chen, M., and K. L. O'Connor. 2005. Integrin α6β4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 24:5125-5130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
