Glucagon activates Ca2+ and Cl- channels in rat hepatocytes
- PMID: 16581855
- PMCID: PMC1779747
- DOI: 10.1113/jphysiol.2006.109819
Glucagon activates Ca2+ and Cl- channels in rat hepatocytes
Abstract
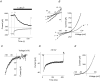
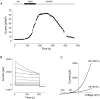
Glucagon is one of the major hormonal regulators of glucose metabolism, counteracting the hepatic effects of insulin when the concentration of glucose in the bloodstream falls below a certain level. Glucagon also regulates bile flow, hepatocellular volume and membrane potential of hepatocytes. It is clear that changes in cell volume and membrane potential cannot occur without significant ion fluxes across the plasma membrane. The effects of glucagon on membrane currents in hepatocytes, however, are not well understood. Here we show, by patch-clamping of rat hepatocytes, that glucagon activates two types of currents: a small inwardly rectifying Ca2+ current with characteristics similar to those of the store-operated Ca2+ current and a larger outwardly rectifying Cl- current similar to that activated by cell swelling. We show that the mechanism of glucagon action on membrane conductance involves phospholipase C and adenylyl cyclase. Contribution of the adenylyl cyclase-dependent pathway to activation of the currents depended on Epac (exchange protein directly activated by cAMP), but not on protein kinase A. The activation of Ca2+ and Cl- channels is likely to play a key role in the mechanisms by which glucagon regulates hepatocyte metabolism and volume.
Figures

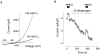






References
-
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Meth. 1994;51:107–116. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Berry MN, Edwards AM, Barritt GJ. High-yield preparation of isolated hepatocytes from rat liver. In: Burdon R H, van Knippenberg PH, editors. Laboratory Techniques in Biochemistry and Molecular Biology. Vol. 21. Amsterdam: Elsevier; 1991. pp. 15–81.
-
- Birnbaum MJ, Fain JN. Activation of protein kinase and glycogen phosphorylase in isolated rat liver cells by glucagon and catecholamines. J Biol Chem. 1977;252:528–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

