Startle responses elicited by whiplash perturbations
- PMID: 16581859
- PMCID: PMC1779749
- DOI: 10.1113/jphysiol.2006.108274
Startle responses elicited by whiplash perturbations
Abstract
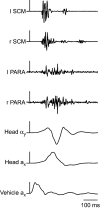
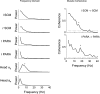
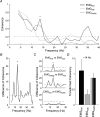
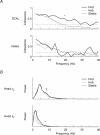
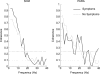
The human startle response produces muscle contractions throughout the body but the most brisk and synchronized contractions appear in the neck muscles. This response, which is greatest with the first exposure to a startling stimulus, could produce excessive and inappropriately directed muscle contractions that could explain the higher incidence of whiplash injuries in people who are unprepared for the collision. This study seeks neurophysiological evidence of startle responses in the neck muscles of 120 healthy subjects exposed to between 1 and 16 rear-end impacts or forward perturbations of different speeds. Startle responses were quantified by the synchronous electromyographic (EMG) activity between 10 and 20 Hz in bilaterally homologous sternocleidomastoid, scalene and cervical paraspinal neck muscles. Coherence analyses of EMGs from the left and right muscles were used to estimate synchrony for: (i) the first unexpected trial, (ii) subsequent habituated trials, and (iii) the superposition of habituated trials and a loud acoustic stimulus (40 ms, 124 dB sound). The peak in coherent EMG activity between contralateral muscle pairs in the 10-20 Hz bandwidth was related to startle. Synchrony in this bandwidth was observed between the left and right muscles during the first impact or whiplash-like perturbation. This synchrony decreased significantly in the habituated trials, but reappeared when the loud acoustic stimulus was introduced. Its presence in the first trial indicates that startle is part of the neuromuscular response to an unexpected rear-end impact. This startle component of the neuromuscular response could play a role in the aetiology of whiplash injuries.
Figures

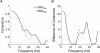
References
-
- Abrahams VC, Rose PK. Projections of extraocular, neck muscle, and retinal afferents to superior colliculus in the cat: their connections to cells of origin of tectospinal tract. J Neurophysiol. 1975;38:10–18. - PubMed
-
- Allum JH, Honegger F, Keshner EA. Head-trunk coordination in man: Is trunk angular velocity elicited by a support surface movement the only factor influencing head stabilization? In: Berthoz A, Graf W, Vidal PP, editors. The Head-Neck Sensory Motor System. New York: Oxford University Press; 1992. pp. 571–575.
-
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Meth. 1997;73:69–79. - PubMed
-
- Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine. 2005;30:E86–E91. - PubMed
-
- Bisdorff AR, Bronstein AM, Gresty MA. Responses in neck and facial muscles to sudden free fall and a startling auditory stimulus. Electroencephalogr Clin Neurophysiol. 1994;93:409–416. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

