Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice
- PMID: 16581908
- PMCID: PMC1421336
- DOI: 10.1073/pnas.0511324103
Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice
Abstract
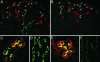
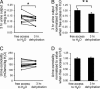
Aquaporin-2 (AQP2) is the predominant vasopressin-regulated water channel in kidney connecting tubule (CNT) and collecting duct (CD) and is essential for renal regulation of body water balance. However, the relative role of AQP2 to urinary concentration in the CNT and CD segments is unknown. To examine this directly, transgenic mice expressing AQP2 selectively in CNT but lacking AQP2 expression in CD (AQP2-CD-KO) and mice lacking AQP2 globally (AQP2-total-KO) were generated by exploiting the Cre/loxP technology. LoxP sites were inserted into AQP2 introns 2 and 3, and transgenic mice were bred with strains expressing Cre recombinase under the control of CD-specific Hoxb7- or global EIIa promoter. Mice lacking AQP2 globally died postnatally (days 5-12). AQP2-CD-KO mice were viable to adulthood and showed decreased body weight, 10-fold increased urine production (0.96 +/- 0.11 vs. 0.10 +/- 0.01 ml/g of body weight), and decreased urinary osmolality (170 +/- 19 vs. 1,630 +/- 135 milliosmoles/kg of H(2)O). Immunohistochemical staining of AQP2-CD-KO kidneys (n = 12) revealed sustained, strong AQP2 expression in CNT cells, whereas >95% of CD principal cells were completely AQP2-negative. Water deprivation for 3 hours caused only marginal decreased urine output (87 +/- 7% of levels when mice had free water access; P = 0.04) with no change in urine osmolality, revealing an absence of compensatory mechanisms. These results demonstrate that AQP2 in CNT is sufficient for postnatal survival and that AQP2 in CD is essential for regulation of body water balance and cannot be compensated for by other mechanisms.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

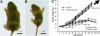



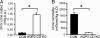
References
-
- Marples D., Knepper M. A., Christensen E. I., Nielsen S. Am. J. Physiol. 1995;269:C655–C664. - PubMed
-
- Sabolic I., Katsura T., Verbavatz J. M., Brown D. J. Membr. Biol. 1995;143:165–175. - PubMed
-
- Yamamoto T., Sasaki S., Fushimi K., Ishibashi K., Yaoita E., Kawasaki K., Marumo F., Kihara I. Am. J. Physiol. 1995;268:C1546–C1551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

