Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses
- PMID: 16582931
- PMCID: PMC7097367
- DOI: 10.1038/nrmicro1389
Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses
Abstract
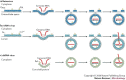
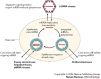
Viruses are divided into seven classes on the basis of differing strategies for storing and replicating their genomes through RNA and/or DNA intermediates. Despite major differences among these classes, recent results reveal that the non-virion, intracellular RNA-replication complexes of some positive-strand RNA viruses share parallels with the structure, assembly and function of the replicative cores of extracellular virions of reverse-transcribing viruses and double-stranded RNA viruses. Therefore, at least four of seven principal virus classes share several underlying features in genome replication and might have emerged from common ancestors. This has implications for virus function, evolution and control.
Conflict of interest statement
The author declares no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

